Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Gaseous and liquid statesA mixture of chlorobenzene and water immiscible is heated and boiled at 1 atm Assuming that the vapors behave like the ideal gas determine at what temperature the Mix and calculate the molar composition of the distillate No 1 2 Nombre Antoine p bar T K A B C Agua 5 40221 1838 67 31 737 Clorobenceno 4 11083 1435 675 55 124 A B and C are the Antoine ecuation constants for Water 1 and chlorobenzene 2 with coccure in ar and Tamn in

Physical Chemistry
EnergeticsA cylinder of height 500 mm and diameter 50 mm is held vertically height wise in a room where the temperature of air is 30 C If the cylinder is maintained at a constant temperature of 90 C calculate rate of heat transfer from the cylinder to surrounding air

Physical Chemistry
ElectrochemistryThe dissolution reactions of gold Au in 1M HNO and 1M aqua regia 1M HNO 1M HCI are given below Au s NO3 aq 4 H aq AU aq NO g 2 H 0 1 Au s NO3 aq 4H aq 4 Cl aq AuCl Reduction Half Reaction 3 Au 3e Au AuCl 3 e Au 4 Cl NO3 4H 3 e NO 2H O 2 H 0 2eH 2 H O 9 a Calculate E cell and Key for the dissolution of gold in 1M HNO under standard conditions 9 aq E V 1 36 1 00 0 96 1 NO g 2 H O n 2 Celalets Et and K for the dissolution of gold in aqua regia under standard conditions 3

Physical Chemistry
GeneralWith the standardized solution what is the corrected established relationship a 10mL Na25203 solution 1232 mg L DO b 10ml Na25203 solution 0 224 mg L DO c 10ml Na25203 solution 1 856 mg L DO d 10ml Na25203 solution 0 104 mg L DO

Physical Chemistry
EnergeticsCalculate the energy required to heat 1 70 kg of ammonia from 48 1 C to 62 1 C Assume the specific heat capacity of ammonia under these 1 Conditions is 4 70 J g K 1 Round your answer to 3 significant digits 0 0 H

Physical Chemistry
GeneralFor a chemical reaction at 324K the values of AH and AS are 64 5 kJ and 183 5 J K respectively a Find the value of AG at 324K 10 Pts b If enthalpy and entropy don t depend on the temperature at what temperature the AG is equals to 0 10 Pts

Physical Chemistry
General14 Draw the organic product expected from each of the following reactions Be sure to indicate stereochemistry where appropriate and to include stereoisomers if any 8 pts NaOCH H C H H C Br CH CH3 SN2 CH OH

Physical Chemistry
GeneralVapour density of following reaction at equilibrium X g 3 Y g is found to be 10 when 1 mole of X is taken in 1 L flask The degree of dissociation of X is Atomic mass of X is 30 O 0 5 0 25 O 0 75 O 0 1

Physical Chemistry
Chemical BondingConsider the following interaction of atomic orbitals no f Hoof a b d J S 8 8 Out of the above combination J S e c 8 88 J S X Number of non bonding combinations Y Number of combinations that produce only antibonding molecular orbital Z Number of combinations that produce only bonding molecular orbital V Number of combinations that produce bonding molecular orbital W Number of combinations that produce antibonding molecular orbital The value of X V 7 Wis

Physical Chemistry
ElectrochemistryThe emf of the given cell is 0 46 V Pt H H PO4 0 4M HPO4 6 4 10 M M 0 81M M s 1 atm 1 Lsolution If E M 2 M 0 76 VI log 3 0 477 log 2 0 3010 and temperature is 25 C The pH of left compartment initially is

Physical Chemistry
GeneralFind the n factor of reactant in the following chemical changes i KMnO4 H Mn2 ii KMnO4 iii KMnO4 OH concentrated basic medium v C O2 CO vii Fe O3 FeSO4 Mn6 iv K Cr O H O Mn H Cr vi FeSO4 Fe O3

Physical Chemistry
Chemical kineticsThe activation energy of the reaction A B C D 38 kcal is 20 kcal What would be the activation energy of the following reactio C D A B 1 20 kcal 2 20 kcal 3 18 kcal

Physical Chemistry
SolutionsThe solubility of salicylic acid at 10 C is 0 14 g per 100 mL of water Based on the total amount of water or other solutions added for the hydrolysis precipitation and filtration of salicylic acid estimate the amount in grams of salicylic acid that was lost in the filtrate for this step of the reaction Assume that the solution was at 10 C during filtration 40 mL were used

Physical Chemistry
GeneralA reaction is run in a bomb calorimeter in which 0 100 mole of X4Y10 is combusted according to the reaction X4Y10 l 6 5 O2 g 4 XO2 g 5 Y 0 e The heat capacity of the calorimeter is 4 2 kJ C and the temperature of the 1 1 L of water in the calorimeter increased in temperature from 25 2 C to 28 3 C Calculate AH at SATP for this reaction and report your answer in kJ mol Density of water is 1 g mL

Physical Chemistry
EnergeticsThe relation between faradic current and time in AC polarography is O if is directly proportional with time O if is inversely proportional to time if is proportional to the square root of time O none of above

Physical Chemistry
GeneralAt 25 C Ksp for PbBr2 is equal to 8 x 10 If the salt is 80 dissociated what is the solubility of PbBr in mol litre 1 3 1 1 60 2 3 4 10 4 1 6 1 6 10 5 1 6 1 6 10 4 71 3 0 8 0 8 71 3 10 5 1 6 1 6 71 2

Physical Chemistry
Nuclear chemistryThe uranium currently present on Earth consists of two isotopes 238U at 99 28 and 235U at 0 72 The half lives are 4 51x109 and 7x108 years respectively Calculate the time that has elapsed since the relative abundance in nature of each isotope was 50 Relative abundances are given in atomic percentage

Physical Chemistry
GeneralWhen an immiscible liquid A was steam distilled with water it gave 200 ml of distillate which contained 55 mL of A The boiling point for distillation was found to be 98 C at a pressure of 755 torr At this temperature the vapour pressure of water was 700 torr The density of the liquid A is 2 g mL Select the correct statement s Assume density of water is 1 g ml Vapour pressure of pure A PA at this temperature is 55 torr Mass of A in distillate is less than mass of water Mass of water in distillate is 145 g Molar mass of A is 173 8 g mol approx

Physical Chemistry
General63 At certain temperature compound AB2 g dissociates according to the reaction 2AB g B g 2AB g With degree of dissociation a which is small compared with unity the expression of Kp in terms of a and initial pressure P is 3 Pa b 3 a P 2 3 pa 3 c P d 2 Pa 2

Physical Chemistry
Energeticsof enthalpy of combusti NCERT Pg 11 1 C H6 g O g 2CO g 3H O 1 2 C s O g CO g diamond 3 C s O g CO g graphite 2

Physical Chemistry
Solutions3 Consider the following graph depicting of total vapour pressure and vapour pressure of components of a solution with mol fraction v p po A XB 0 A B po B XB 1 XB D mol fraction Which of the following is correct regarding the plot 1 A is more volatile than B 2 B will be more in liquid phase starting from equal mole fractions of A and B 3 AD BD CD 4 AD BC CD

Physical Chemistry
Surface chemistryusing the data in the graphs determine the gibbs free energy entropy and enthalpy for the mechanical stretching data graphs thanks Mass kg Mass kg 0 141 0 14 0 139 0 138 0 137 0 136 0 135 305 0 082 0 0815 0 081 0 0805 0 08 0 0795 y 0 0002x 0 0805 R 0 9895 310 Rubber Band Elastomer Box 305 310 315 y 6E 05x 0 0996 R 0 9966 315 y 0 0002x 0 081 R 0 986 320 Temperature K 325 Spring Box 320 y 7E 05x 0 1032 R 0 986 325 330 y 0 0002x 0 0807 R 0 9834 330 335 y 6E 05x 0 1004 R 0 991 335 Trial S Trial 6 Trial 7 Linear Trial 5 Linear Trial 6 Linear Trial 7 Trial 4 Trial 5 Trial 6 Linear Trial 4 Linear Trial 5 Lincar Trial 6

Physical Chemistry
GeneralOFFICE OF CHIEF W All the students of 1 II III IV year who are staying in the hostels are advised to go to the class on time During the class hours if students are present in the hostel and not attending the class college management will take appropriate action This has the consent of Principal Jandl Dr Jagadish R S Chief Warden CC to Principal CAO AO Registrar Deans All HODs TPO Hostel Superintendents caretakers Parents Notice Board

Physical Chemistry
EquilibriumAt 650 K the reaction MgCO3 s MgO s CO2 g has an equilibrium constant Kp 0 026 A 10 0 L container at 650 K has 1 0 gram MgO s and a CO2 g pressure of 0 0260 atm The container is then compressed to 100 It 15 1611

Physical Chemistry
Chemical BondingAnswer ANY 10 question from Q39 to Q50 1x10 10 Read the passage given below and answer the following questions Ionization Energy In chemistry Ionization energy is also called as ionization potential Ionization energy is the energy required to remove an electron from a gaseous atom or ion It is denoted by Ei The first or initial ionization energy or Ei of an atom or molecule is the energy required to removeone mole of electrons from one mole of isolated gaseous atom or ion It is quantitatively expressed as X energy Where X is any atom or molecule capable of ionization X is that atom or molecule with an electron removed and e is the removed electron This is generally an endothermic process Generally the closer the outermost electron are to the nucleus of the atom the higher the atoms or elements ionization energy In these questions Q No 39 to 41 a statement of Assertion followed by a statement of reason is given 39 Assertion A Ionization potential ofBe is greater than that of B Reason R The first electron released from Be is from ns orbital while it is from np orbital in B a Assertion and reason both are correct statements and reason is correct explanation for Assertion b Assertion and reason both are correct statements and reason is not correct explanation for Assertion Assertion is correct statement but reason is wrong statement d Assertion is wrong statement but reason is correct statement

Physical Chemistry
Chemical BondingVolume the number of particles and temperature are all held constant Which statement s best describes the speed distribution curve for the blue heavy particles compared to the speed distribution curve for the red light particles More than one statement is correct The shape of the speed distibution curves are similar The speed distribution curve for the blue heavy particles is wider indicating that the blue particles exhibit a large range of speeds The speed distribution curve for the red lighter particles is wider indicating that the red particles exibit a larger range of speeds The most probable speed peak for the blue heavy particles is located farther to the left indicating that the blue particles are slower thar the red particles The most probable speed for the red light particles is located farther to the left indicating that the red particles are faster than the blue particles

Physical Chemistry
Solutions4 A gas exerts a partial pressure of 0 2 atm over water The Henrys law constant is 104 atm The solubility of the gas in millimol per litre will be approximately 1 2 75 10 5 3 1 1 x 10 5 2 1 1 10 3 4 27
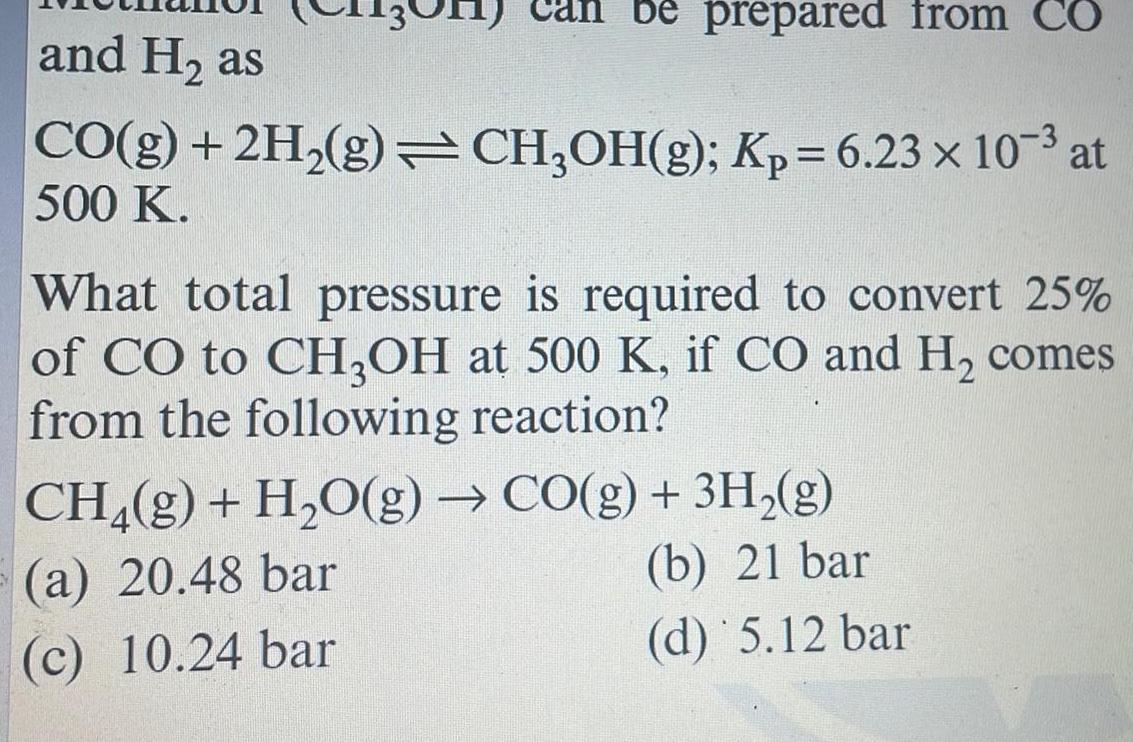
Physical Chemistry
Generalbe prepared from CO and H as CO g 2H g CH OH g Kp 6 23 x 10 at 500 K What total pressure is required to convert 25 of CO to CH3OH at 500 K if CO and H comes from the following reaction CH g H O g CO g 3H g a 20 48 bar b 21 bar c 10 24 bar d 5 12 bar

Physical Chemistry
Solutions3 The total vapour pressure of a solution of methanol and ethanol in an ideal solution is given by P 135 100 where a mole fraction of methanol Pure state vapour pressure of methanol is 1 235 mm g 3 35 mm q 2 100 mm g 4 135 mm g

Physical Chemistry
Solid stateSublimation energy of Ca is 121 kJ mol Also dissociation energy of Cl is 242 8 kJ mol total ionization energy of Ca g Ca g is 2422 kJ and electron gain enthalpy of Cl is 355 kJ lattice energy of CaCl2 is 2430 S kJ The value of AH for the following process is in kJ mol A 355 kJ mol C 355 J mol B 355 kJ mol D 355 J mol

Physical Chemistry
Atomic StructureAn unielectronic ion of element A atomic number of element A is n has velocity of electron in xth orbit equal to velocity of electron in H atom in nth orbit Group number of element A in modern periodic is if kinetic energy of electron in H atom in xth orbit is more than 0 2125 eV atom and x n 60houl 3 101

Physical Chemistry
Atomic Structureth An unielectronic ion of element A atomic number of element A is n has velocity of electron in x orbit equal to velocity of electron in H atom in nth orbit Group number of element A in modern periodic is if kinetic energy of electron in H atom in xth orbit is more than 0 2125 eV atom and x n Bathoun

Physical Chemistry
SolutionsAssertion At equilibrium vapour phase will be always rich in component which is more volatile Reason The composition of vapour phase in equilibrium with the solution is determined by the partial pressures of the components

Physical Chemistry
General5 If Dr and Do are the theoretical and observed vapour densities at a definite temperature and a be the degree of dissociation of a substance then a in the terms of Do Dr and n number of moles of products formed from 1 mole reactant is calculated by the formula DT Do DT Do n 1 DT n 1 Do a a Do DT 1 n DT b a c a d a H D DT n 1 Dr

Physical Chemistry
Gaseous and liquid statesDuring Test 4 it was determined that the pressure for 100 blue heavy particles and 100 red ligh particles is less than the pressure for 200 blue heavy particles Therefore for an ideal gas the total pressure of a system is independent from particle size Answer 1 less than Answer 2

Physical Chemistry
GeneralAll of the following statements are correct regarding potentiometric titrations except O O O O Liquid junction potentials will not influence the study The emf of the cell is zero at the equivalence point They are suitable for colored or turbid reactions These are not suitable for analysis of dilute solutions less than 0 001 M

Physical Chemistry
Solid stateThree atoms A B and C crystallize in a cubic solid lattice where A atoms are present at the body centre B atoms are present at the edge centre as well as at the corners of the cube and C atoms are present at the face centres of the cube Now if all the atoms are removed from the two 4 fold axis and the one 2 fold axis passing through the cube then the formula of the compound is 1 B C 3 ABC 2 AB C7 4 A C

Physical Chemistry
Electrochemistryd Protein hydrolysate is to be assayed for aspartic acid Exactly 5 0 mg of aspartic acid having a specific activity of 0 46 mCi mg is added to the hydrolysate From the hydrolysate 0 21 mg of highly purified aspartic acid having a specific activity of 0 01 mCi mg can be isolated How much aspartic acid was present in the original hydrolysate fal Write the of Triti 3

Physical Chemistry
Surface chemistry0 1 g of animal charcoal is mixed with 0 25 litres of 0 2 M CH COOH solution If the extent of adsorption 1 m on animal charcoal is 2 then the final molarity of the CH COOH solution left is 1 0 053 M 3 0 196 M 2 0 186 M 40 083 M

Physical Chemistry
Gaseous and liquid states9 0 1 mole of N 204 g was sealed in a tube under one atmospheric conditions at 25 C Calculate the number of moles of NO 2 g present if the equilibrium N 04 g 2NO g Kp 0 14 is reached after some time P a 1 8 x 102 b 2 8 x 10 0 034 d 2 8 x 10 2

Physical Chemistry
GeneralSuppose 12 9 g of lead II acetate is dissolved in 150 mL of a 0 50 Maqueous solution of ammonium sulfate Calculate the final molarity of lead II cation in the solution You can assume the volume of the solution doesn t change when the lead II acetate is dissolved in it Be sure your answer has the correct number of significant digits M 10

Physical Chemistry
Atomic Structure59 Which of the following is correct for num electrons number of orbitals and type of c respectively in N orbit 1 4 4 and 8 3 32 16 and 4 2 4 8 and 16 4 4 16 and 32 not of four quon

Physical Chemistry
GeneralRepeat 10 00 mL aliquots of a CAM solution with a molarity of 0 04 were titrated using a NaOH solution The mean titration volume of the NaOH solution for an acceptable set of titrations was 12 26 mL Calculate the molarity of the NaOH solution State the answer to four decimal places and without units

Physical Chemistry
SolutionsIf a patient is receiving 155 mL of 5 00 m v glucose solution every 3 0C hours how many grams of glucose does the patient receive in 14 0 hours Select the appropriate items from the menus to show how to calculate the answer using dimensional analysis and then select the correct answer 14 0 hours 14 0 hours 100 mL 100 mL

Physical Chemistry
General10 For the following mixture of oils ver Cottonseed oil Cetyl alcohol 2 0 g Calculate RHLB and the quantity of surfactants to prepare 100 mL of an or emulsion RHLB 10 5 18 0 g Use a mixture of Span 20 HLB 8 6 and Tween 60 HLB 14 9 This mixtur should amount to 2 of the formula Span 20 1 4g Tween 60 0 6g

Physical Chemistry
Nuclear chemistryPhosphorus 32 is a commonly used radioactive nuclide in biochemical research particularly in studies of nucleic acids The half life of phosphorus 32 is 14 3 days What mass of phosphorus 32 is left from an original sample of 175 mg of Na332PO4 after 30 5 days Assume that the atomic mass of 32P is 32 0

Physical Chemistry
Equilibriumogen gas and lodine gas react via the ing equ Kc 79 at 650 K If 0 031 mol HI is placed in an empty 1 0 L flask at 650 K what are the equilibrium concentrations of HI I2 and H M M M HI ba 6 IH Z 6 1 6 H
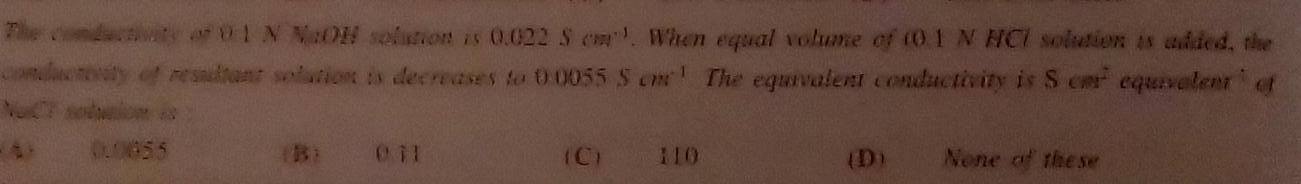
Physical Chemistry
GeneralThe conductivity of 01 N NaOH solution is 0 022 S When equal volume of 0 1 N HCI solution is added the conduceaty of resultant solution is decreases to 00055 S cm The equivalent conductivity is Scar equivalent of 00855 Bi 011 None of the se

Physical Chemistry
GeneralPhase sensitive AC polarography technique depends on the following fact O O O O Voltage Lags Current by 90 Capacitive Current lags Voltage by 90 O Faradic Current lags Voltage by 90 Faradic Current lags Capacitive

Physical Chemistry
Equilibrium2NH3 g 61 N g 3H g For the reaction initially the mole ratio was 1 3 of N H At equilibrium 50 of each has reacted If the equilibrium pressure is P the partial pressure of NH3 at equilibrium is P 3 a b P 4 c P 6 d P 8