Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Chemical BondingThe molecular orbitals of 1 3 butadiene not in proper order are given below f1 f2 f3 f4 are the 2p orbitals on carbon atoms 0 372f1 0 602f2 0 602f3 0 372f4 V2 0 602f 0 372f2 0 372f3 0 602f4 V3 0 372f1 0 602f2 0 602f3 0 372f4 V4 0 602 1 0 372f2 0 372f3 0 602f4 The correct order of the orbitals with increasing energy is A C V1 V2 V3 V4 V3 V4 V1 V2 B D W3 V2 V4 V1 V4 V3 V2 V1

Physical Chemistry
Chemical kineticsThe overall reaction of hydroxide radical with hydrogen gas occurs as follows OH g H g H O g H g This reaction plays a significant role in the chemistry of the earth s atmosphere by eventually converting OH to HO2 through the subsequent reaction of H with O HO2 is involved in the destruction of stratospheric ozone J Phys Chem A 2006 110 6978 The rate constants for the reaction of OH with H were found to be 1 21x107 L mol 1 s1 at 79 C and 3 43 106 L mol 1 s1 at 14 C Part A Determine the activation energy for the reaction of OH with H2 reaction O 16 3 kJ mol 1 O 21 2 kJ mol 1 O 17 6 kJ mol 1 O21 2 kJ mol 1 16 3 kJ mol 1 Submit Request Answer

Physical Chemistry
Chemical kineticsThe activation energy in kJ m chemical reaction whose rate become doubles on increasing the temperature from 298 K to 308 K will be NCERT Pg 118 1 46 21 3 52 89 2 61 28 4 71 89

Physical Chemistry
GeneralFor the cell reaction Mg s 2 Ag aq Mg2 aq 2 Ag s E 3 17 V at 298 K The standard Gibb s free energy cell change for the cell reaction is O 611 8 kJ mol O 514 2 kJ mol O 870 2 kJ mol 290 4 kJ mol

Physical Chemistry
Equilibrium3 2 8 106 4 2 8 10 How many grams of CaC 0 will dissolve in 58 one liter of saturated solution Kp of CaC 04 is 2 5x 10 and its molecular weight is 128 1 0 0064 g 3 0 0032 g 2 0 0128 g 4 0 0640 g The solubility of AgCl in pure water is 103 M 59 3 2 8 x 10 In fac a 0 yam af CaC O K 2 50 128 at 1 0 0064 g 3 0 0032 g 4 2 8 10 AUCH 2 0 0128 g 4 0 0640 g

Physical Chemistry
Solid stateIron exhibits bcc structure at room temperature Above 900 C it transforms to fcc structure The ratio of density of iron at room temperature to that at 900 C assuming molar mass and atomic radii of iron remains constant with temperature NEET UG 2018 A G 3 2 3 3 4 2 B D 4 3 3 2 1 2

Physical Chemistry
GeneralThe conjugate base of H PO4 is 1 H PO 2 H PO4 3 HPO 4 PO ALLEN 86 H POgre 1 H PO4 3 HPO 2 H PO4 4 PO

Physical Chemistry
GeneralThe infrared spectrum of a diatomic molecule exhibits transitions at 2143 0 cm and 4260 0 cm corresponding to excitations from the ground state to first and second vibrational states respectively The value of the fundamental frequency cm for the molecule is B 2169 M A 2156 C 2182 2195

Physical Chemistry
ElectrochemistryA small amount of a non volatile non electrolyte solute is dissolved in 56 8 cm of benzene density 0 889 g cm At room temperature the vapour pressure of this solution is 98 88 mm Hg while that of benzene is 100 mm Hg Find the molality of this solution A 0 144 m 14 4 m C 1 44 m 0 100 m B D

Physical Chemistry
Solid stateWhen NaCl molten is dopped with 105 mol 54 of AICI what is the concentration of cation vacancies 1 6 02 10 6 mol 2 12 04 107 mol 3 6 02 10 7 mol 4 12 04 106 mol NaCl 10 AICI f at aft 1 6 02 x 10 mol 2 12 04 10 7 mol 3 6 02 10 7 mol 4 12 04 10 6 mol d

Physical Chemistry
SolutionsIf 1 71 of sugar molar mass 342 are dissolved in 500 ml of an aqueous solution at 300 K what will be its osmotic pressure 1 Why the freezing point depression of 0 4 M NaCl solution is nearly twice than that of 0 4 M glucose solution

Physical Chemistry
Solid stateAB crystallizes in a body centred cubic lattice with edge length a equal to 387 pm The distance between two oppositely charged ions in CBSE PMT 2010 the lattice is A 335 pm C 200 pm B 250 pm D 300 pm

Physical Chemistry
GeneralA certain saturated hydrocarbon effuses about half as fast as methan The molecular formula of the hydrocarbon a compound made up c only carbon and hydrogen is a C4H8 c C3H6 b C4H10 d C3H8

Physical Chemistry
Solutionshow to solve equiva 6H20 mol AND calculated mass of Ca 103 2 6H2O grams Mass of Ca 10 6H O before transfer to flask g Mass of Ca 103 6H Oafter transfer to flask g Net mass of Ca 10 6H O 9 Final burette reading mL Initial burette reading mL Volume of Na S O solution used L Mol of Na S O mol Equivalent mol of Ca 10 6H 0 mol Calculated mass of Ca 10 6H 0 g Percentage purity of Ca 10 103 2 Titration 1 0 1755 0 0078 35 42 0 21

Physical Chemistry
Equilibrium3 5 3 4 6 75 Calculate pH of 0 1 M solution of NH CI The 54 dissociation constant of NH OH is 2 10 1 5 15 2 6 15 3 7 4 8 85 3 5 3 4 6 75 0 1 M NH CIH OH farise ferdian a 10 1 5 15 2 6 15 3 7 4 8 85

Physical Chemistry
Atomic StructureIf a particle of mass 1 mg is moving with a velocity of 400 ms then the de Broglie wavelength of the moving particle will be h 6 6 x 10 34 Js 1 65 x 10 32 m 1 65 x 10 33 m 1 65 10 30 m 1 65 10 36 m

Physical Chemistry
Nuclear chemistryThe failure of classical wave theory to account the distribution of energy in the spectrum of black bod radiation was due to the assumption that radiation energy is a continuous b discrete c a mixture of continuous and discrete d electromagnetic

Physical Chemistry
GeneralIn lead chamber process for the manufacture of H SO NO is the homogenous catalyst 2SO O 2SO3 formation of the intermediate is indicated by 1 Brown coloured vapours 3 Violet coloured vapours 2 Green coloured vapours 4 Light yellow coloured vapours

Physical Chemistry
SolutionsThe vapour pressure of benzene at 80 C is lowered by 10 mm by dissolving 2 g of a non volatile substance in 78 g of benzene The vapour pressure of pure benzene at 80 C is 750 mm The molecular mass of the substance will be g mol A 15 C 1500 LOBT LB 150 D 2000

Physical Chemistry
Atomic StructureTYPE questions Each question has 4 choices 1 Read More Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule Which of the following has the highest dipole moment O CO2 O HI O H 0

Physical Chemistry
Equilibrium4 HNO K 10 Which of the following salts will not hydrolyse 56 2 Na PO 4 Ba CIO4 2 1 NH4 SO4 3 CH COOK 1 NH4 SO4 3 CH COOK 2 Na PO 4 Ba CIO4 2

Physical Chemistry
GeneralThe solubility of electrolytes MX MX and 60 fa electrolytes MX MX MX MX is 1 x 103 moles per litre Hence their factar 1 x 10 respective solubility products are 1 10 4 x 10 27 10 2 2 10 4 x 10 27 10 2 X 3 10 8 x 108 32 10 2 4 None of these factual qu 1 10 4 x 10 27 10 2 2 10 4 10 27 10 2 3 10 8 x 108 32 10 2 4 tolo nepap

Physical Chemistry
Chemical kineticsThe rate constant for the decomposition of a certain substance is 1 70 x 10 2 dm mol 1 sec1 at 27 C and 2 x 10 2 dm mol 1 sec1 at 37 C If order of reaction is x and Arrhenius parameter of reaction is y dm mol sec 1 then y 1000 is Given In 1 176 0 162 R 8 3 43x J mol K e4 86 129

Physical Chemistry
Atomic StructureAccording to VBT which of the following overlapping results pi bond covalent bond in 0 molecule formation when z axis is internuclear axis 1 2s 2s 1 III 1s 1s V 2pz 2pz Question Type Single Correct Type 2 II V 3 II IV II 2px 2px IV 2py 2py 4 IV V

Physical Chemistry
GeneralTwo moles of an equimolar mixture of two alcohals R OH and R OH are esterified with one mole of acetic acid If only 80 of the acid is consumed till equilibrium an the quantities of ester formed under equilibrium are in the ratio 3 2 What is the valu of equilibrium constant Kc for the esterification of R OH A 0 48 B 3 69 C 2 2 D 4 61

Physical Chemistry
GeneralConduction in a p type semiconductor is increased by a increasing the band gap b decreasing the temperature c adding appropriate electron deficient impurities d adding appropriate electron rich impurities
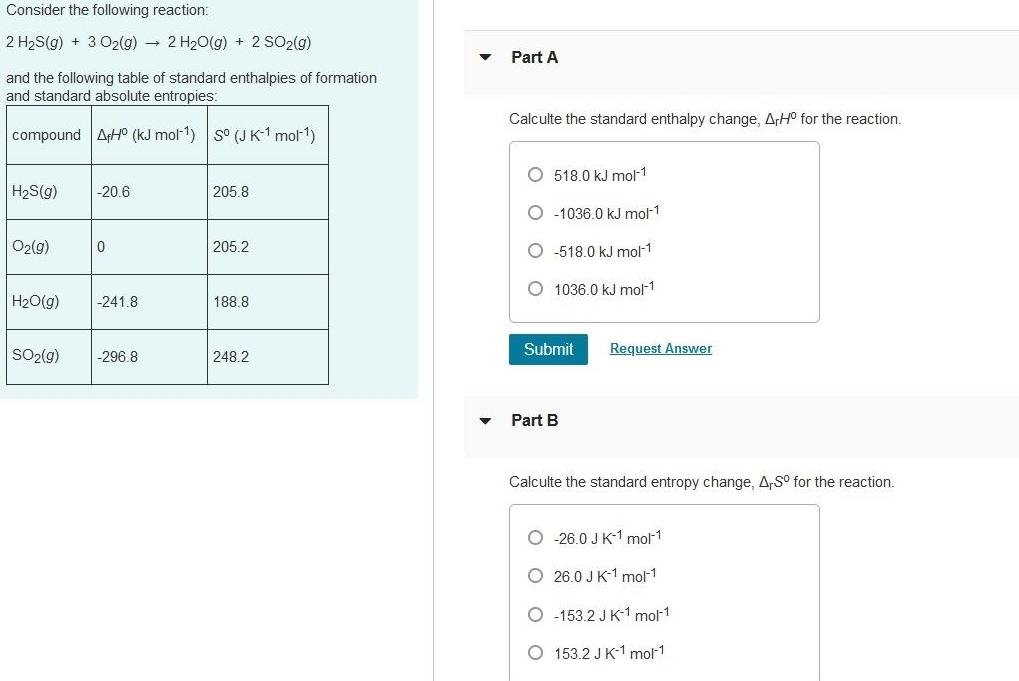
Physical Chemistry
EnergeticsConsider the following reaction 2 H S g 3 O2 g 2 H O g 2 SO2 g and the following table of standard enthalpies of formation and standard absolute entropies compound AHO kJ mol 1 S J K 1 mol H S g O2 g H O g SO g 20 6 0 241 8 296 8 205 8 205 2 188 8 248 2 Part A Calculte the standard enthalpy change ArH for the reaction O518 0 kJ mol 1 O 1036 0 kJ mol 1 O 518 0 kJ mol 1 O 1036 0 kJ mol 1 Submit Request Answer Part B Calculte the standard entropy change A S for the reaction O 26 0 J K 1 mol 1 O26 0 J K 1 mol 1 O 153 2 J K 1 mol 1 O 153 2 J K 1 mol 1

Physical Chemistry
Chemical kineticsHalf life period of a first order reaction is 10 min Starting with initial concentration 12 M the rate after 20 min is KCET 2015 A 0 0693 M min B 0 693 x 3 M min 1 C 0 0693 x 3 M min D 0 0693 x 4 M min 1

Physical Chemistry
Atomic Structure4 Which of the following is correctly matched Orbital a 4 f b 4 s c 3 d d 3 p Ans c Nodal property 2 radial node 0 nodal plane 2 nodal plane 3 radial node Hint Number of nodal plane 1 Number of nodal plane of 3d orbital 2

Physical Chemistry
GeneralHeat of dissociation of CH COOH is 0 005 kcal g hence enthalpy change when I mol of Ca OH is completely neutralised by CH COOH is a 27 4 kcal c 26 8 kcal b 13 6 kcal d 27 1 kcal

Physical Chemistry
Chemical kinetics9 The kinetic data for the given reaction k A g 2B g table for three experiments at 300 K Ex No A M 0 01 0 02 0 02 1 2 3 C is provided in the following B M Initial rate M sec 0 01 6 930 10 6 0 01 0 02 5 1 386 x 107 1 386 x 10 5 In another experiment starting with initial concentration of 0 5 M and 1 M respectively for A and B at 300 K The rate of reaction after 50 minutes from start of experiment in M sec is x 10 5 The value of x is

Physical Chemistry
SolutionsDensity of 15 by weight H SO4 solution is 1 5 g mL then molarity of solution is Question Type Single Correct Type 1 2 3 1 02 M 2 29 M 1 176 M

Physical Chemistry
General2 Sodium bicarbonate on heating decomposes to form sodium carbonate CO and water If 0 2 moles of sodium bicarbonate is completely decomposed how many moles of sodium carbonate is formed J K CET a 0 1 b 0 2 c 0 05 d 0 025

Physical Chemistry
Solid stateIn a face centred cubic lattice atom A occupies the corner positions and atom B occupies the face centre positions If one atom of B is missing from one of the face centred points the formula of the compound is AIEEE 2011 A A B3 C A B BA B5 D AB

Physical Chemistry
Atomic StructureFor H atom the energy required for the removal of electron from various sub shells is given as under 3s 3p 3d 1 0 0 The order of the energies would be 2 Question Type Single Correct Type 3 4 E E2 E3 E3 E2 E E E E E2 E3 None of these n E3 0

Physical Chemistry
GeneralOne mol of an organic compound A containing C H N on reaction with excess HNO liberates 44 8 litres of N N T P and on reaction with ammoniacal AgNO gives a white ppt containing 108 gm of silver Two moles of A eaction with excess MeMeBr produces methane gas whose volume measured at N T P will be B 112 litre A 224 litre C 89 6 litre D 179 2 litre

Physical Chemistry
Solid stateConsider a cube containing in unit cells of a cubic system A plane ABCD obtained by joining the mid points of the edges on one of its identical faces had atoms arranged as shown Let p be the packing fraction Choose the correct option 22 A n 1 P 212 C n 8 p B n 8 p D n 1 p 11 3 28 D A B Arrangement of atoms

Physical Chemistry
Surface chemistry3 1 2 cal K 4 2 77 cal K Which of the following is an example of 56 f homogeneous catalysis reaction NO g A 2SO g O2 g 2SO3 g B Hydrolysis of aqueous sucrose solution in presence of aqueous mineral acid C CH COOCH H O t CH COOH CH OH D CO g 2H g 1 A C 3 A D Cu ZnO Cr O s 2 A B C 4 A B D HCI 1 3 1 2 cal K CH OH A 2SO g O g B face 43427 4 2 77 cal K NO g 1 A C 3 A D 2SO3 g C CH COOCH H O CH COOH E CH OH D CO g 2H g Cu ZnO Cr O3 s 2 A B C 4 A B D CH O

Physical Chemistry
Equilibrium2 CuS will ppt 3 Both ZnS and CuS will ppt 4 Both Zn and Cu will remain in solution Solubility of AgBr will be minimum in 1 Pure water 2 0 1 M CaBr 3 0 1 M NaBr 4 0 1 M AgNO 1 ZnS will ppt 2 CuS will ppt 3 Both ZnS and CuS will ppt 4 Both Zn and Cu will remain in sol AgB va 2 0 1 M CaBr 3 0 1 M NaBr 4 0 1 M AgNO

Physical Chemistry
Atomic StructureMg Which one of the following set of quantum numbers is not possible for 4p electron 2019 1 a n 4 7 1 m 1 m 2 b n 4 1 m 0 m S c n 4 1 m 2 m d n 4 1 m 1 m N N

Physical Chemistry
EquilibriumGiven that K for water is 10 3 M at 62 C 75 compute the sum of pOH and pH for a neutral aqueous solution at 62 C 1 7 0 2 13 30 3 14 0 4 13 0 62 C Ja fa fac H H K10 3 M 1 62 C T 1 7 0 2 13 30 3 14 0 4 13 0

Physical Chemistry
General84 ALLEN Which of the following does not give a 84 disproportion reaction 1 P NaOH 2 S NaOH

Physical Chemistry
EquilibriumALLEN Sum of H O and OH in an aqueous solution 76 of NaCl at 25 C is 1 2 x 10 4 M 3 10 4 M 2 2 107 M 4 10 M 25 C NaCl H O OH 1 2 10 4 M 3 10 4 M 0 H 2 2 107 M 4 107 M

Physical Chemistry
Solid state3 For a reaction consider the plot of ln k versus 1 T given in the figure If the rate constant of this reaction at 400 K is 10 5s then the rate constant at 500 K a 10 4 1 c 10 6 s l In k Slope 4606 K 1 T b 4 x 10 4 S d 2 x 10 4 S

Physical Chemistry
GeneralEnthalpy of solution of BaCl and BaCl 2H O are x and y kJ mol respectively The enthalpy of hydration of BaCl to BaCl 2H O is 1 x y kJ 2 y x kJ 3 x y kJ 4 x y kJ

Physical Chemistry
Gaseous and liquid statesO and SO is filled in two different containers A and B respectively at same T and P A has circular orifice while B has square orifice of edge length equal to the diameter of the orifice of vessel A then ratio of rate of diffusion of the gases from vessel A to that from vessel B will be A 2 2 B C 2 1 4 D 2

Physical Chemistry
Gaseous and liquid statesTO Tayum Tyvivituly virusiv TVm60 A mixture of 2 moles of A and 2 mole of B forms and ideal solution The vapour pressure of solvents A and B are 200 mm of Hg 400 mm of Hg respectively then find mole fraction of A and B in vapour phase What are the values of AHmix and AVmix of ideal gas

Physical Chemistry
Solid stateNa and Mg crystallize in bcc and fcc type crystals respectively then the number of atoms of Na and Mg present in the unit cell of their respective crystal is A 4 and 2 C 14 and 9 B DY 9 and 14 2 and 4

Physical Chemistry
GeneralComprehension 3 Electronegativity is a feature which is relative and depends on several factor like oxidation numbe hybridizations etc For instance the electronegativity of differently hybridized carbon are sp sp 2 sp 39 10 2 99 2 66 2 48 Which of the following contain all carbon of equal electronegativity A Propene B Propadiene C Cyclopropene Which of the following is correct H C CH H C CH HC CH c a b A The C C abond is of equal strength in a and b B The C C a bond is stronger in a than c D Cyclopropane

Physical Chemistry
Solid stateIn a cubical close packing structure of mixed oxide the lattice is made of oxide ion 20 of the tetrahedral voids are occupied by X ion and 50 of octahedral voids are occupied by Y ion The formation of the oxide is Only one correct answer A X2Y203 B XY204 C X4Y5010