Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Surface chemistryConsider the chemisorption of N on a metal surface Identify the correct statement O The value of AH is always positive e are transferred from metal to 2p orbital of N molecule Due to adsorption of N on metal bond order between N N increases After adsorption the energy required to dissociate the N N bond will be lesser as compared to isolated N molecule

Physical Chemistry
EquilibriumTo 100 ml of an aqueous solution of 0 1 M CH COOH Ka 2 10 0 01 mol of HC1 g is passed Select correct options regarding the resulting solution A Degree of dissociation of acetic acid in resulting solution is 10 4 pH of resulting solution is nearly 1 S Degree of dissociation of water in resulting solution is 1 8 10 5 D Concentration of OH ions contributed by water is resulting solution is 10 7 M

Physical Chemistry
Chemical BondingConsider the following reaction xMnO4 yC 0 zH Z xMn 2yCO H O The values of x y and z in the reaction are respectively JEE Main 2013 1 5 2 and 16 3 2 5 and 16 2 2 5 and 8 4 5 2 and 8

Physical Chemistry
Solid stateA solid metal crystallises in ABCABC type closest packing F C C in three dimension If the density of element and it s atomic radius are 5g cm and 100 2 respectively the molar mass in gm mole of element is NA 6 x 1023

Physical Chemistry
SolutionsSolution A B C and D have ph values equal t o 12 7 4 6 5 respectively 1 Which solution will evolved with ammoni a with ammonia chloride Why 2 Which solution will evolved with co3 with Na2co3 Why 3 What type of reaction occurs between sol ution a and and c

Physical Chemistry
General1 Haemoglobin contains 0 334 of iron by weight The molecular weight of haemoglobin is approximately 67200 The number of iron atoms Atomic weight of Fe is 56 present in one molecule of haemoglobin is 1 4 2 6 3 3 4 2

Physical Chemistry
Chemical kinetics72 How many of the following is not correct 1 1 2 of zero order reaction is proportional to initial concentration of reactant ii 2 of first order reaction is independent of initial concentration of reactant iii t 2 of zero order reaction is equal to R 2K log2 k iv 1 2 of first order reaction is equal to v First order reactions will never go for completion

Physical Chemistry
Chemical Bonding9 How many moles of oxygen gas will be evolved when 24 5 g of KCIO is heated for complete decomposition Molar mass of KCIO 122 5 g KCIO 1 0 1 2 0 2 3 0 3 3 A AKCI 0 2

Physical Chemistry
Gaseous and liquid statesIIT JEE 4 An equal volume of a reducing agent is titrated sepa with 1 M KMnO in acidic neutral and alkaline me The volumes of KMnO4 required are 20 mL in 33 3 mL in neutral and 100 mL in alkaline mediun out the oxidation state of manganese in each red product Give the balanced equations for all the thr reactions Find out volume of 1 MK Cr O consu the same volume of the reducing agent is titrated in medium IIT JEE SEDIA DE n Butane is produced by the monobromination of followed by the Wurtz reaction Calculate the vol ethane at STP required to produced 55 g n butane bromination takes place with 90 yield and the reaction with 85 yield IIT JEE A mixture of H C O4 oxalic acid and NaHC O4 w 2 02 g was dissolved in water and the solution mad L 10 mL of the solution required 3 0 mL of 0 1 N

Physical Chemistry
General1 With reference to Rutherford s Scattering Experiment answer the following questions A What was the thickness of gold foil used i n the experiment B With which particles was the gold foil bo mbarded
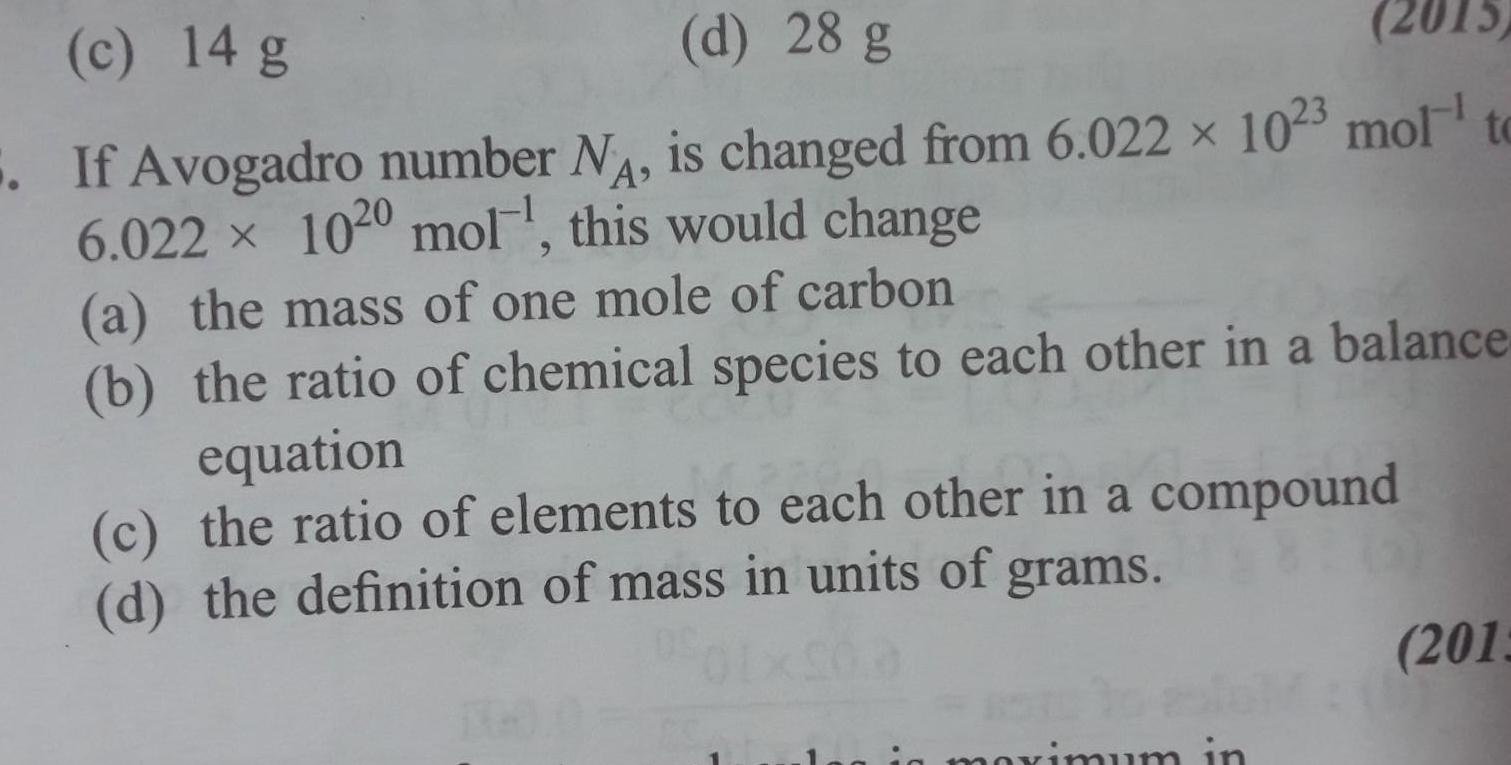
Physical Chemistry
Generalc 14 g d 28 g 2015 If Avogadro number N is changed from 6 022 x 1023 mol to 6 022 1020 mol this would change a the mass of one mole of carbon b the ratio of chemical species to each other in a balance equation c the ratio of elements to each other in a compound d the definition of mass in units of grams maximum in 2013

Physical Chemistry
ElectrochemistryIf the hydrogen electrode is dipped in a solution of pH 2 at 25 C then the reduction potential of the electrode would be 0 118 V 0 084 V 0 059 V

Physical Chemistry
Chemical kinetics67 Rate constant K varies with temperature by equation logk min 5 conclude that A Pre exponential factor A is 105 C Ea is 9 12 k cal 2000 kcal RTX 2 303 we can B Ea is 2 kcal D The pre exponential factor A is 5

Physical Chemistry
EquilibriumConsider the following reaction 2NO 2H N 2H O The given reaction follows the mechanism 1 NO NO N O Fast and reversible II O H N O H O Slow III N O H N H O Fast Which of the following statement is incorrect If the concentration of NO becomes twice then rate of reaction becomes 4 times If the concentration of H becomes twice then rate of reaction becomes 4 times Overall order of reaction is 3 If the concentration of both NO and H becomes thrice then rate reaction becomes 27 times

Physical Chemistry
EnergeticsI have learnt that internal energy U is a state function and it only depends on temperature So if Delta T 0 then Delta U 0 However when I was studying exothermic and endothermic reactions it was written in my textbook that at a constant temperature and pressure Delta U is negative for exothermic reactions How is that possible Shouldn t Delta U be zero since the temperature is constant

Physical Chemistry
EnergeticsConsider the following setup of two bulbs with adiabatic walls separated by an adiabatic valve The left side flask is of volume 1L and contains 0 2 moles of N O4 and 0 1 moles of NO at equilibrium at 25 C N O g 2NO g The larger flask on the right side is of 3L volume and is empty at 25 C The connecting valve is suddenly opened The correct statement s regarding this system is re A Total number of moles after opening the valve is 0 34 B Entropy of the system keeps on increasing till the system regains the equilibrium C The total number of moles remain the same before and after opening of the valve D Ke value for the equilibrium is 20

Physical Chemistry
General2 AH ve AS ve There are many substance for example CO NO etc do not follow third law of thermo dynamics because 1 these substances are polar 2 these substance do not form crystal at absolute zero temperature 3 these substances do not fulfill the perfectly ordered arrangement at absolute zero 4 these substance follow the second law of thermodynamics 3 82 C A 1 2 3 4
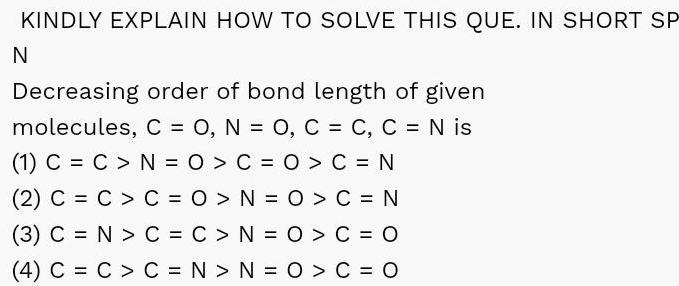
Physical Chemistry
Chemical BondingKINDLY EXPLAIN HOW TO SOLVE THIS QUE IN SHORT SP N Decreasing order of bond length of given molecules C O N O C C C N is 1 C C N O C O C N 2 C C C O N 0 C N 3 C N C C N O C O 4 C C C N N O C O

Physical Chemistry
GeneralHow much product will be formed if each of the reactants used is 100 g in quantity Atomic weight Al 27g mol and Atomic weight of Cl 35 5 g mol Max score 4 Neg score 1 150 g 125 g

Physical Chemistry
GeneralVolume strength of H O is 5 6 V then whic statement is are true 1 1 mL of H O given sample liberate 5 6 mL O at N T P 2 Normality of solution is 1 N 3 Molarity of solution is 2 M 4 7 g of H O2 are present per 1000 mL solution

Physical Chemistry
Chemical kinetics218 PO 11 2 183 sec decay to 82Pb tv2 sec by a emission while Pb2 4 is a B emitter In an experimental starting with 1 mole of pure Po2 8 how much time would be required for the number of nuclei of Pb to reach maximum

Physical Chemistry
EquilibriumVAVILOG G The equilibrium constant for the ionization of R NH g in water as RNH g H O RNH aq OH aq is 8 x 10 at 25 C Find the pH of a solution at equilibrium when pressure of R NH g is 0 5 bar The rate of react

Physical Chemistry
GeneralFind out D in the following sequence of reactions Br Fe Zn HCI D C H B C A CH CH CCI CCI3 Br Br Br
Physical Chemistry
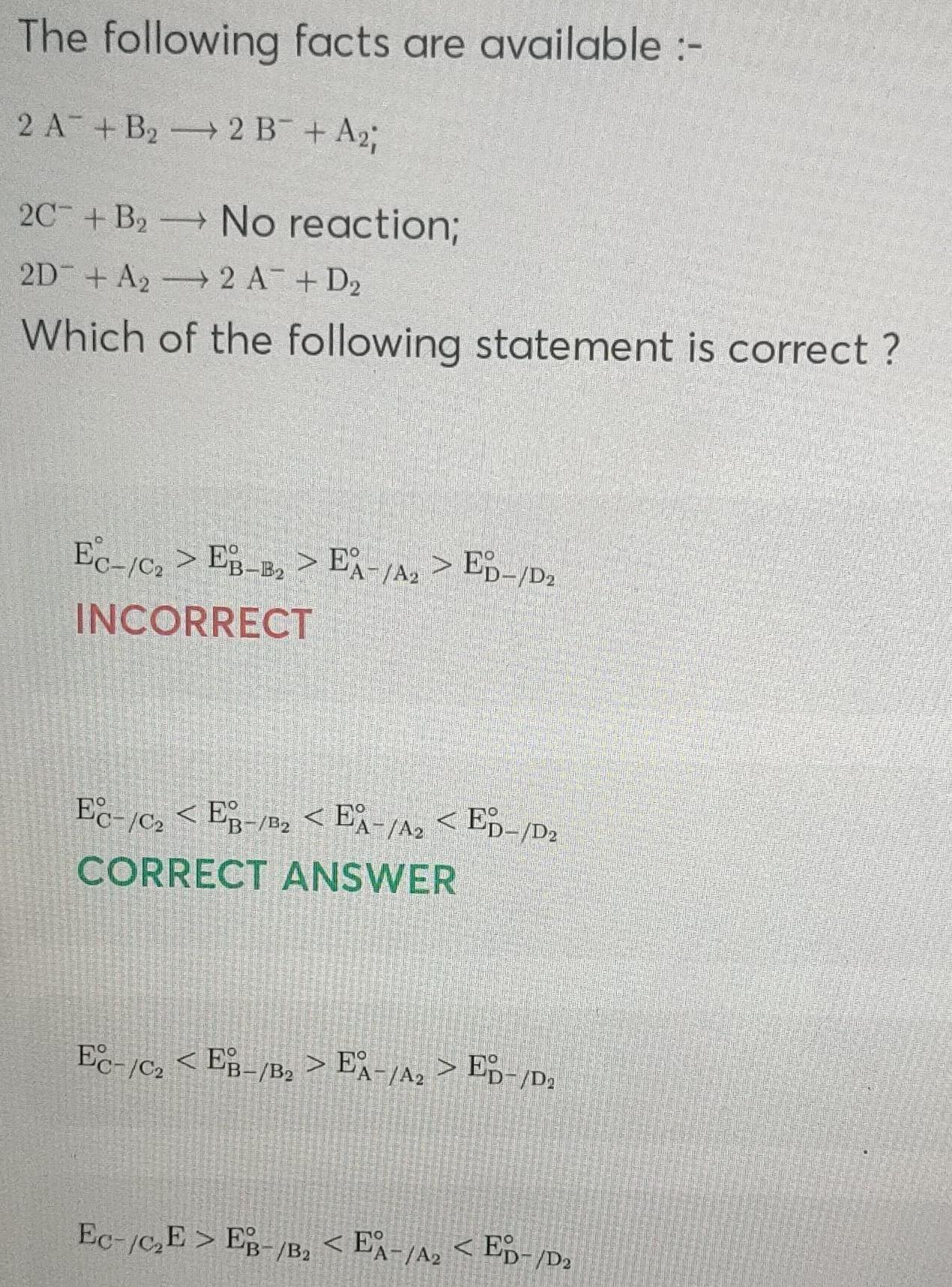
ElectrochemistryThe following facts are available 2 A B 2 B A 2C B No reaction 2D A 2 A D Which of the following statement is correct Ec c EB B EA A ED D INCORRECT EC C2EB B EA A ED D CORRECT ANSWER Ec C EB B EA A ED D Ec C E EB B 2 EA A ED D

Physical Chemistry
EnergeticsO The composition of Liquefied Petroleum Gas LPG is 0 5 ethane 0 1 acetylene 16 4 propane 2 1 ethylene 74 butane and 6 9 butene If the room temperature is at 30 C what is the pressure inside the tank and the composition of the gas that first issues from this mixture

Physical Chemistry
Gaseous and liquid statesA real gas has critical temperature and critical pressure as 40 C and 10 atm respectively then liquification c gas is possible at 1 50 C and 8 atm 3 25 C and 12 atm 2 45 C and 8 atm 4 45 C and 12 atm

Physical Chemistry
Generalsolution of silver nitrate Max score 2 Neg score 0 5 copper will remain unreacted due to the presence of nitrate ions the solution will remain colourless

Physical Chemistry
Atomic Structureestion No 5 40 If a box containing large number of balls identical in each and every asp which statistics can be applied to balls AO B E statistics BOF D statistics CCO No statistics DO M B statistics 20064696 202 4696 2020064 6 2020064696 020064696

Physical Chemistry
General3 H is limiting reagent 9 40 g water is formed Equal volume of N and H react to form ammonia under suitable condition then the limiting reagent is N 3H 2NH3 2 N 4 No one reactant is limiting reagent 1 H 3 NH How many grams of calcium oxide is obtained on heating 100 g of CaCO3 s

Physical Chemistry
General1803080201030001 202 Question No 18 40 The most probable distribution of N indistringuishable particles various energy levels according to the Fermi Dirac statistics 696 20 AO gi 1 BG 1 co Si BON x G 1 6962020064696 2020064 02 969792 20064 90 Si x BT 1 8 DO n x BG 1 2020064696 202006 9691 4696 20

Physical Chemistry
Solutions2 Among the following solution mixtures the one with highest boiling point is A 100ml 0 1M acetic acid and 100ml 0 1M NaCl B 100ml 0 1M CaCl2 and 100ml 0 1M NaCl C 100ml 0 1 M Na2SO4 and 100ml 0 1M BaCl2 D 100ml 0 1 M sucrose and 100ml 0 1M NaCl

Physical Chemistry
Chemical kineticsA substance A gets converted to B following single step mechanism The variation of rate constant k vs temperature T is given by following equation 301 3 logio k 8 T From this information calculate fraction of activated particles at 1000 K Multiply your answer by 10 Given In2 0 693 In 10 2 3

Physical Chemistry
Gaseous and liquid states60 litre of such a mixture The half life of 32p is 14 3 day Calculate the specific activity of a phosphorous containing specimen having 1 0 part per million 32P Atomic weight of P 31 The hydrogen atom in the ground state is excited by mann wavelength A The resulting mmute How many mill

Physical Chemistry
ElectrochemistryResistance of a conductivity cell filled with 0 1 mol L KCl solution is 100 Q If the resistance of the same cell when filled with 0 02 mol L KCl solution is 520 2 calculate the conductivity and molar conductivity of 0 02 mol L KCl solution The conductivity of 0 1 mol L KCE solution is 1 29 S m

Physical Chemistry
Nuclear chemistryIS384 Hg the rate The reaction given below rate constant for disappearance of A is 7 48 x 10 3 sec Calculate the time required for the total pressure in a system containing A at an initial pressure of 0 1 atm to rise to 36 0 145 atm and also find the total pressure after 100 sec KD K k kB kc 2 Ad g 4B g C g a b Aia d t 1 Im A o A E optical rotation of reaction 37

Physical Chemistry
EnergeticsHeat transfer is very important for life s progress as everything needs to gain or lose heat even human beings In the process industry the temperature of input and output streams of any equipment should be adjusted by exchanging the heat between fluids Different types of Heat exchangers are used in process industries for this purpose such as shell and tube heat exchangers Analyze the diagram of the shell and tube heat exchanger shown below to answer the associated questions h PRO b i Heat is transferred by three different methods use your knowledge by explaining the methods of transferring heat and show how it 2 Marks is applying in a heat exchanger

Physical Chemistry
Generaliv Without constructing the actual matrices construct the set of characters of a representation of a point group of the following molecules in the basis set given against each Molecule Ammonia Basis Set The set of unit vectors stretched along the N H bonds

Physical Chemistry
EquilibriumFor the given sequential reaction A kiBk2 C Initial concentration of A is 20M Calculate the approximate concentration of C after 10 if k 2 108 min k 0 0693min 1

Physical Chemistry
Solutions2 Colligative properties depend on O The nature of the solute particles dissolved in solution O The number of solute particles in solution O The physical properties of the solute particles dissolved in solution O The nature of solvent particles

Physical Chemistry
Chemical kineticsa 16 1 c 1 4 b 2 1 d 4 1 2015 Cancelled 1 What is the mass of the precipitate formed when 50 mL of 16 9 solution of AgNO3 is mixed with 50 mL of 5 8 NaCl solution Ag 107 8 N 14 0 16 Na 23 C1 35 5 a 3 5 g b 7 g c 14 g d 28 g 2015 If Avogadro number NA is changed from 6 022 1023 mol to

Physical Chemistry
Surface chemistry3 Which of the following has the least flocculating value for a sol whose particles move towards the cathode in the presence of an electric field 1 NaCl 3 Na PO 2 Na SO4 4 Na C O4

Physical Chemistry
Energeticsbenzer i mole HEMISTRY The The combustion of takes place at 21Sk flatm After combustion CO g and H O 1 are produced and 3267 0 kJ of heat is liberated Calculate the standard enthalpy of formation A H of benzene Standard enthalpies of formation of CO g and H O 1 are 393 5 kJ mol and 285 83 kJ mol respectively
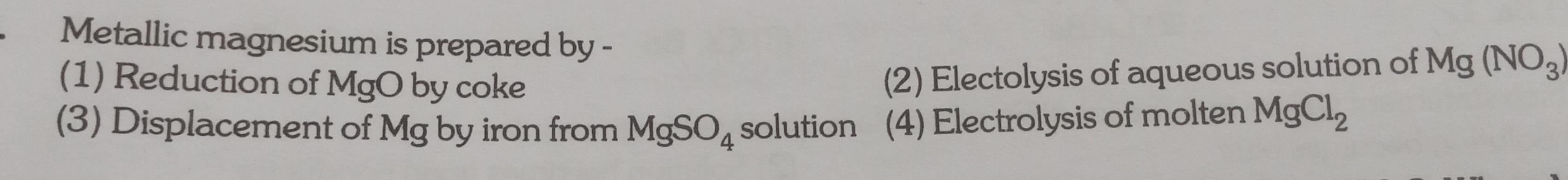
Physical Chemistry
GeneralMetallic magnesium is prepared by 1 Reduction of MgO by coke 3 Displacement of Mg by iron from MgSO4 solution 4 Electrolysis of molten MgCl 2 Electolysis of aqueous solution of Mg NO3
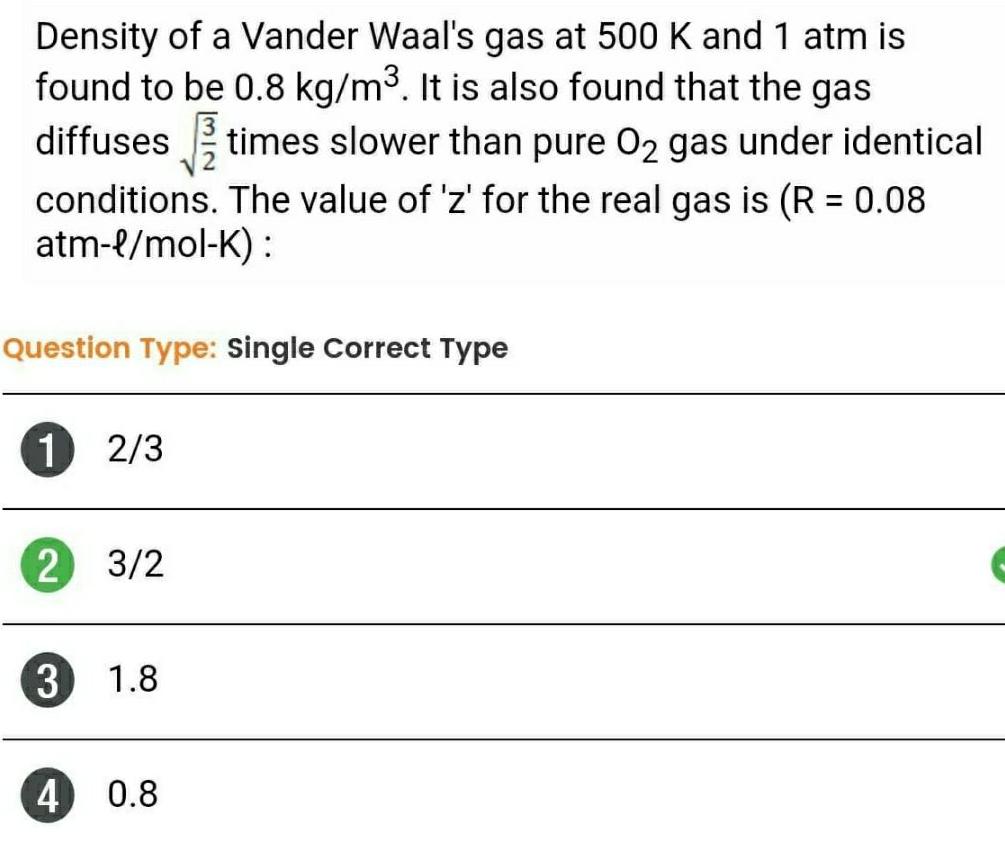
Physical Chemistry
Gaseous and liquid statesDensity of a Vander Waal s gas at 500 K and 1 atm is found to be 0 8 kg m It is also found that the gas diffuses times slower than pure O gas under identical conditions The value of z for the real gas is R 0 08 atm l mol K Question Type Single Correct Type 1 2 3 2 3 2 3 4 1 8 0 8

Physical Chemistry
Chemical kineticsThe pressure of a gas decomposing at the surface of a solid catalyst has been measured at different times and the results are given below t sec 0 100 200 4 103 Pr Pascal 3 5 10 3 10 Determine the order of reaction its rate constant 300 2 5 10

Physical Chemistry
Solutions4 Mixture of volatile components A and B has total vapour pressure in torr P 254 119 A total where XA is the mole fraction of A in mixture Hence p and pare in torr 1 254 119 3 135 254 2 119 254 4 154 119

Physical Chemistry
Chemical kineticsIf an equimolar mixture of the two radioact substances having decay constant 5 303 hr and 3 hr respectively decay simultaneously Then ratio of nuclides 2nd with respect to 1st at the end of 2 hr is 10 P The value of P is Given In A 90 1 2 303 log Al

Physical Chemistry
Chemical kinetics56 What will be the order of reaction for a chemical change having logt 2vsloga where a initial concentration of reactant t 2 half life A Zero order log 2 B First order log a C Second order D None of these

Physical Chemistry
Generalwhen iron spoon is electroplated with copper which of these represent reduction reaction skipped B C D Cu aq 2e Cu s Cu s Cu aq 2e Fe aq 2e Fe s Fels Fe aq 2e

Physical Chemistry
General4 The values of Ksp of CaCO3 and CaC204 are 4 7 10 and 1 3 x 10 9 respectively at 25 C If the mixture of these two is washed with water what is the concentration of Ca2 ions in water 1 7 746 10 5 M 3 6 856 10 5 M 2 5 831 x 10 5 M 4 3 606 10 5 M