Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Chemical kineticsThe Enthalpy of formation of H SO4 at 298K will be Given S 02 SO SO2 1 2 02 SO3 SO3 H O H SO4 AH 300 KJ AH 100 KJ AH 130 KJ H 1 2 02 H O AH 280 KJ Question Type Single Correct Type 1 2 810 KJ 710 KJ

Physical Chemistry
Generaldt dt dt a a EXAMPLE 5 When 50 ml of 2M solution of N O5 was heated 0 28 L of O at NTP was formed after 30 mi Calculate the concentration of N O5 at that time and also find the average rate of reaction

Physical Chemistry
Nuclear chemistrySingle 1 Bombardment of aluminium of a particle leads to its artificial disintegration in two ways i and ii as shown below Product X Y and Z respectively are 14Si30 X i 134127 1 Proton neutron positron 2 Neutron positron proton 3 Proton positron neutron 4 Positron proton neutron 15 P30 Y Si 0 Z 14

Physical Chemistry
Energetics5 In comparison to a 0 01 M solution of glucose the depression in freezing 1 poin point of a 0 01 M MgCl2 solution is O The same O About twice O About three times O About six times 4

Physical Chemistry
ElectrochemistryThe following items are obtained from the stockroom for construction of a galvanic cell two beakers and a salt bridge wire with clips 200 mL of a 1 00 M Mn2 aq solution 200 mL of a 1 00 M Au3 aq solution manganese and gold electrodes a Draw a galvanic cell constructed from the items listed above Label the anode and the cathode and show the direction of the electron flow on your drawing

Physical Chemistry
General28 At temperature above 1073 K coke can be used to reduce Fe0 to Fe How can you justify this reduction with Ellingham diagram s Using Ellingham diagram we observe that at temperature greater than 1073 K We know that according to Ellingham diagram compound having lower A GS undergo its formation AGIC CO AG Fe FeO Hence coke can reduce FeO to Fe

Physical Chemistry
General2012 2 A closed vessel with rigid walls contains 1 mol of 238U and 92 1 mol of air at 298 K Considering complete decay of 238U 92 to 206Pb the ratio of the final pressure to the initial pressure 82 of the system at 298 K is

Physical Chemistry
Chemical Bonding3 A solution containing 2 675 g of CoCl2 6NH3 molar mass 267 5 g mol is passed through a cation exchanger The chloride ions obtained in solution were treated with excess of AgNO3 to give 4 78 g of AgCl molar mass 143 5 g mol The formula of the complex is atomic mass of Ag 108 u 1 Co NH3 6 C13 3 CoCl NH3 3 2 CoCl NH3 4 CI 4 COCI NH3 Cl2

Physical Chemistry
GeneralA solid compound X on heating gives CO gas and a residue The residue mixed with water forms On passing an excess of CO through Y in water a clear solution Z is obtained On boiling Z compour X is reformed The compond X is 1 CaCO3 2 Na CO 3 K CO 4 Ca HCO3 2

Physical Chemistry
GeneralBe sure to answer all parts Give the oxidation number of sulfur in the following a SOCI select b H S select c H SO3 select d Na S select TINTOFF 4 3 2

Physical Chemistry
EquilibriumH g 1 g 2HI g in equilibrium for the forward reaction is 167 kJ mol 1 whereas for the reverse reaction is 180 kJ mol The presence of catalyst lowers the activation energy by 80 kJ mol Assuming that the reactions are made at 27 C and the frequency factor for forward and backward reactions are 4 104 and 2 10 3 respectively calculate K wold

Physical Chemistry
General37 The density of argon face centered cubic cell is 1 83 g cm at 20 C What is the length of an edge a unit cell Atomic mass Ar 40 b 0 569 nm a 0 599 nm c 0 525 nm 38 The density of nickel face centered cubic cell is 8 94 g cm3 at 20 C wh d 0 551 nm 1 1

Physical Chemistry
Chemical kineticsi i Rate Concentration What is the order of the reaction What is the unit of rate constant K for the reaction Derive an expression to calculate the time required for completion of the zero orde

Physical Chemistry
EquilibriumIf pH and percentage degree of hydrolysis for the salt BA 0 1M are x and y respectively then x y is equal to given K HA 106 K BOH 106 a xMnO vPbO zHNO HMnO Pb NO H O then minimum integer

Physical Chemistry
General4 4 0 m 1 solution of H O2 completely reacts with 10ml of 0 5 molar KMnO4 solution o specific gravity 0 4316 as per reaction 2KMnO4 3H SO4 5H2O2 K2SO4 2MnSO4 502 8H O If volume strength of H O2 is x then find the value of 3x 7

Physical Chemistry
Solid statea 12 0 b 6 94 44 An element X At mass 80 g mol has fcc structure Calculate no of unit cells in 8 gm of X a 0 4 NA b 0 1 NA c 4 NA d none of these Molybdenum At mass 96 g mol crystallizes as bcc crystal If density of crystal is

Physical Chemistry
Chemical Bonding0201 stion No 15 40 The intrinsic viscosity of a water soluble protein at 25 C is fo 180 cm g The concentration of the solution of this protein in water at it will have a relative viscosity of 1 5 will be AO 0 2 78 10 g cm 3 BO 1x10 g cm 3 S CO1 78x10 g cm3 1 23 10 g cm3 269 90 020064696 2026 064696 20200646 9697900202 969

Physical Chemistry
Equilibrium53 For the reaction A g 2B g C g D g K A B Initial pressure of A and B are respectively 0 60 atm and 0 80 atm At a time when pressure o C is 0 20 atm rate of the reaction relative to the initial value is C A 6 B dx 1 48 D 1 24

Physical Chemistry
EquilibriumSlope of the graph between log Keq and 1 T Tin Kelvin is 400 Which of the following change will ncrease the concentration of B g in an equilibrium mixture of A B and C 1 Addition of a gas which reacts with only A 2 Addition of inert gas at constant pressure 3 Increase in temperature at constant volume 4 Decrease in volume at constant

Physical Chemistry
Solutionsing point of an aqueous solution of benzoic acid is 101 C A solution of equal molality was prepared by dissolving benzoic acid in zene Then identify correct statements from the following ven that for water K 0 83k kg mol K 1 86k kg mol and for benzenek 2 0k kg mol and boiling point is 75 C Freezing point of the aqueous solution of benzoic acid is 2 24 C Boiling point of the solution of benzoic acid in benzene is 77 4 C Observed molar mass of benzoic acid in water is equal to its value in presence of benzene as the solvent Observed molar mass of benzoic acid in water is less than to its value in presence of benzene as the solvent

Physical Chemistry
GeneralIdentify the incorrect match Name A Unnilunium B Unniltrium C Unnilhexium D Unununnium a A i c C iii IUPAC Official Name i Mendelevium ii Lawrencium iii Seaborgium iv b B ii d D iv NEET 2020 Darmstadtium

Physical Chemistry
Solid stateINCORRECT statements regarding defects in crystalline solids are Frenkel and Schottky defect are stoichiometric defects Metal excess defect always cause decrease in density of the ionic solid and increase in its electrical conduct Metal deficiency defects always cause increase in density and electrical conductivity of the ionic solids Impurity defects creates cationic vacancies in the ionic solids

Physical Chemistry
Solutions1 2 5 L 0 0025 M weak acid CH3COOH PKA 4 7 is added with 7 5 L 0 003 M CH3COOH PKA 4 7 what is the pH for the mixture Using numeric methods 2 f 2 5 L weak acid HOCI PKA 7 6 is found to have a pH of 4 5 what is HOCI concentration After 2 5 L water is added what is the pH now Using numeric methods

Physical Chemistry
Chemical kineticsRead the paragraph carefully and answer the The rate constant of reaction is related with T by Arrhenius equation Ea RT k A e B 3 Where k rate constant A Pre exponential factor or frequency factor Ea RT Fraction of molecules that is present in activated state ecules e E Activation energy Fraction of molecules that can cross the activation energy increases with increase in temperature or with decrease in activation energy conc Find the maximum rate constant A 3 46 x 102 day C 10 day SOLVE QUESTION 3 not 1 20 days Time R P Follows the above conversion graph and this first order reaction occurring at 27 C then if 102 molecules are in activated state 10 B 3 46 x 102 day D can t be determined In the question 1 of reaction is radioactive disintegration What should be its half life at 600 C A 20 days B 20 days

Physical Chemistry
Surface chemistryWhich one of the following statements is wrong about adsorption 1 It is a selective and specific process 2 It is a reversible process 3 An increase in the gaseous adsorbate causes an increase in a adsorption However at high pressure the adsorption becomes constant 1 It is an endothermic process

Physical Chemistry
ElectrochemistryThe standard free energy of formation of AgCl s at 25 C is 109 7 kJ mol and H Cl aq is 131 2 kJ mol Find E of a cell made up with standard hydrogen electrode and Cl Ag AgCl s A B C D 0 23 V 0 45 V 0 90 V 0 35 V

Physical Chemistry
ElectrochemistryAn electric current of 0 2F is passed through 2 lit of 0 25M CuSO 4 solution using pt electrodes After electrolysis the molarity of the solution is assume that there is no change in the volume of solution

Physical Chemistry
Chemical kineticsThe following data are for the following decomposition of ammonium nitrite in aqueous solution Vol of N in cc 6 25 9 00 11 40 13 65 33 05 The order of reaction is a zero c two Time min 10 15 20 25 Infinity b one d three

Physical Chemistry
Atomic StructureAn atomic orbital X has 2 radial nodes but onlyone angular node An another atomicorbital Y has oneradial node but two angular nodes The correct information s regarding these orbitals is are C D X is 4p orbital Y is 4d orbital When an electron jumps fromorbital X to orbital Y inH atom energy is absorbed When an electron jumps fromorbital Y to orbital X inHe atom energy is released

Physical Chemistry
GeneralFind the total number of different possible combination product Considering only combination of radical but not disproportionation of radical for following Wurtz reaction Na H3C CI H3C CH CI H3C CH CH CI Dry ether Backspace

Physical Chemistry
EquilibriumWhen AgCl is treated with a NH3 solution part of the precipitate dissolves to form a complex ion What weight of AgCl will dissolve in 1L of 1M NH3 solution Data ksp AgCl 1 82 10 10 B log 2 7 1 SOLUTION 6 86 gl 1

Physical Chemistry
GeneralQ 55 The density of solid Argon is 1 6 ml at 233 C If the Argon atom is assumed to be sphere of radius 1 5 x 108 cm then the of solid Argon is apparently occupied Take N 6 x 1023 Atomic mass of Ar 40

Physical Chemistry
Gaseous and liquid statesvi A process engineer decided to use the distillation process to separate a liquid mixture consists of two components the boiling point of each compound is the same which is 80 C do you agree with this decision Justify the 2 marks reason

Physical Chemistry
Chemical Bonding0 1 g of each of compound A and B both A and B are non dissociative and non volatile but at the boiling point A dissociates into 2C 40 degree of dissociation and B dissociates into 2D 60 dissociation solute dissolved in 100 g of solvent The increase in boiling point is found to be 0 11 K and depression in freezing point is found to be 0 3 C If Kf and Kb of the given solvent is 2 K molal and 0 5 K molal respectively identify the correct statement s Molar mass of B Molar mass of A Molar mass of B Molar mass of A 2 3 Relative lowering in vapour pressure at the boiling point is 0 35 if molar mass of solvent is 20 g mol and 10 g of each A and B is taken in 100 g solution Relative lowering in vapour pressure at the boiling point is 0 35 if molar mass of solvent is 30 g mol and 10 g of each A and B is taken in 100 g

Physical Chemistry
Chemical Bonding50 A substance was known by its mode of synthesis to contain 10 atoms of carbon per molecule along with unknown number of atoms of chlorine hydrogen and oxygen Analysis showed 60 5 carbon 5 55 hydrogen 16 10 j oxygen and 17 9 chlorine The Empirical formula of the compound is 1 C 0H OCl 3 C10H10OCI 2 C 0H 1O Cl 4 C 0H 2O Cl

Physical Chemistry
Chemical BondingWhich of the following intermolecular forces are not responsible for liquid state of Bromine at room temperature Dipole dipole forces Clear Resp London dispersion forces Hydrogen bonds

Physical Chemistry
GeneralA 2 650x10 2 M solution of glycerol C3H O3 in water is at 20 0 C The sample was created by dissolving a sample of C3Hs Os in water and then bringing the volume up to 1 000 L It was determined that the volume of water needed to do this was 998 9 mL The density of water at 20 0 C is 0 9982 g mL
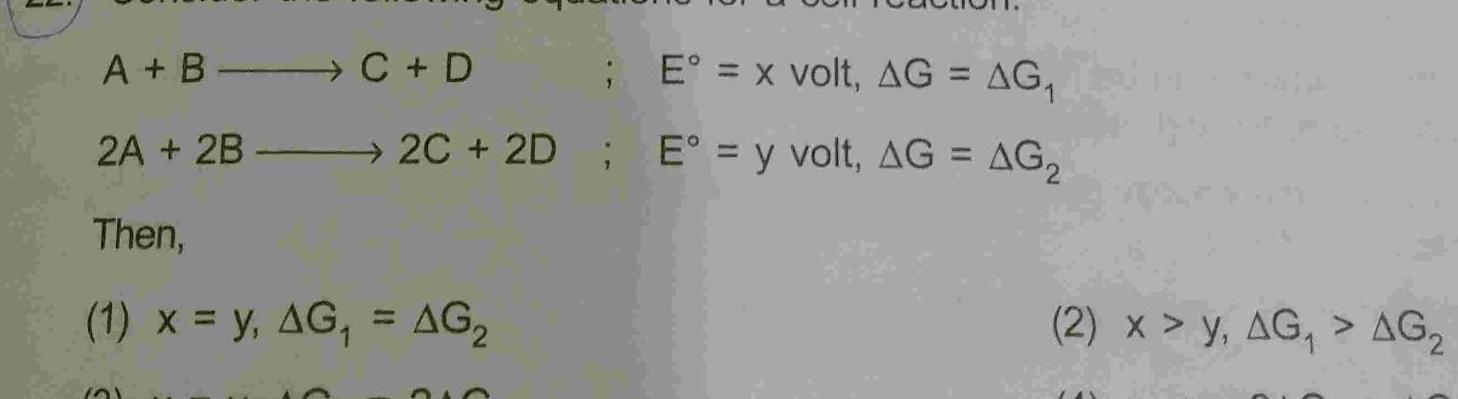
Physical Chemistry
GeneralA B C D 2A 2B Then E x volt AG AG 2C 2D E y volt AG AG 1 x y AG AG 21 210 2 x y AG AG

Physical Chemistry
Gaseous and liquid statesAt critical temperature pressure and volume The compressibility factor Z is 1 3 0 3 3 4 2 4 50 3 3 00 phos that of No O permanent ga

Physical Chemistry
Solutions3M M 37 Consider the following reaction M MOMO M2 a 2x 24 if 1 mol of MO oxidises 1 67 mol of M to b then the value of x in the reaction is b 3 c 4 2 4 5 38 In the mixture of NaHCO and Na CO the volum given HCl required is x ml with phenolphthalein in and further y mL required with methyl orange ind Hence volume of HCI for complete reaction of Nam e b y 9 10 mL of NaHCO is oxidised by 10 mL of 0 MnO Hence 10 mL of NaHCO is neutralised a 10 mL of 0 1 M NaOH b 10 mL of 0 02 M N e 10 mL of 0 1 M Ca OH d 10 mL of 0 05 N Ba 2

Physical Chemistry
Solutions10 The vapour pressure of a pure liquid A is 40 mm Hg at 310 K The vapour pressure of this liquid in a solution with liquid B is 32 mm Hg The mole fraction of A in the solution if it obeys Raoult s law is 1 0 8 3 0 2 2 0 5 4 0 4
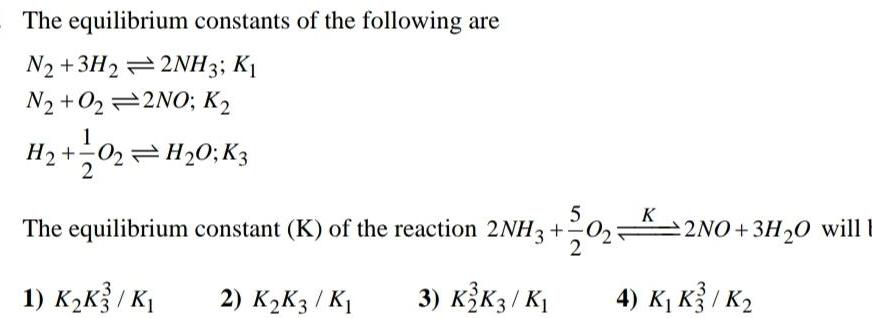
Physical Chemistry
EquilibriumThe equilibrium constants of the following are N2 3H22NH3 K1 N2 O22NO K H 1 0 3 02 H 0 K3 5 The equilibrium constant K of the reaction 2NH3 2 1 K K3 K 2 K K3 K 3 K2 K3 K K 2NO 3H O will E 4 K K3 K

Physical Chemistry
Chemical Bondinga Which formula of noble gas species is isoelectronic with IBr2 b Halogens have maximum negative electron gain enthalpy in the respective periods the periodic table Why c Sulphur hexafluoride is used as a gaseous electrical insulator Explain d SF6 is known but SnCl4 is not known Explain e Molecular nitrogen N is not particularly reactive Explain
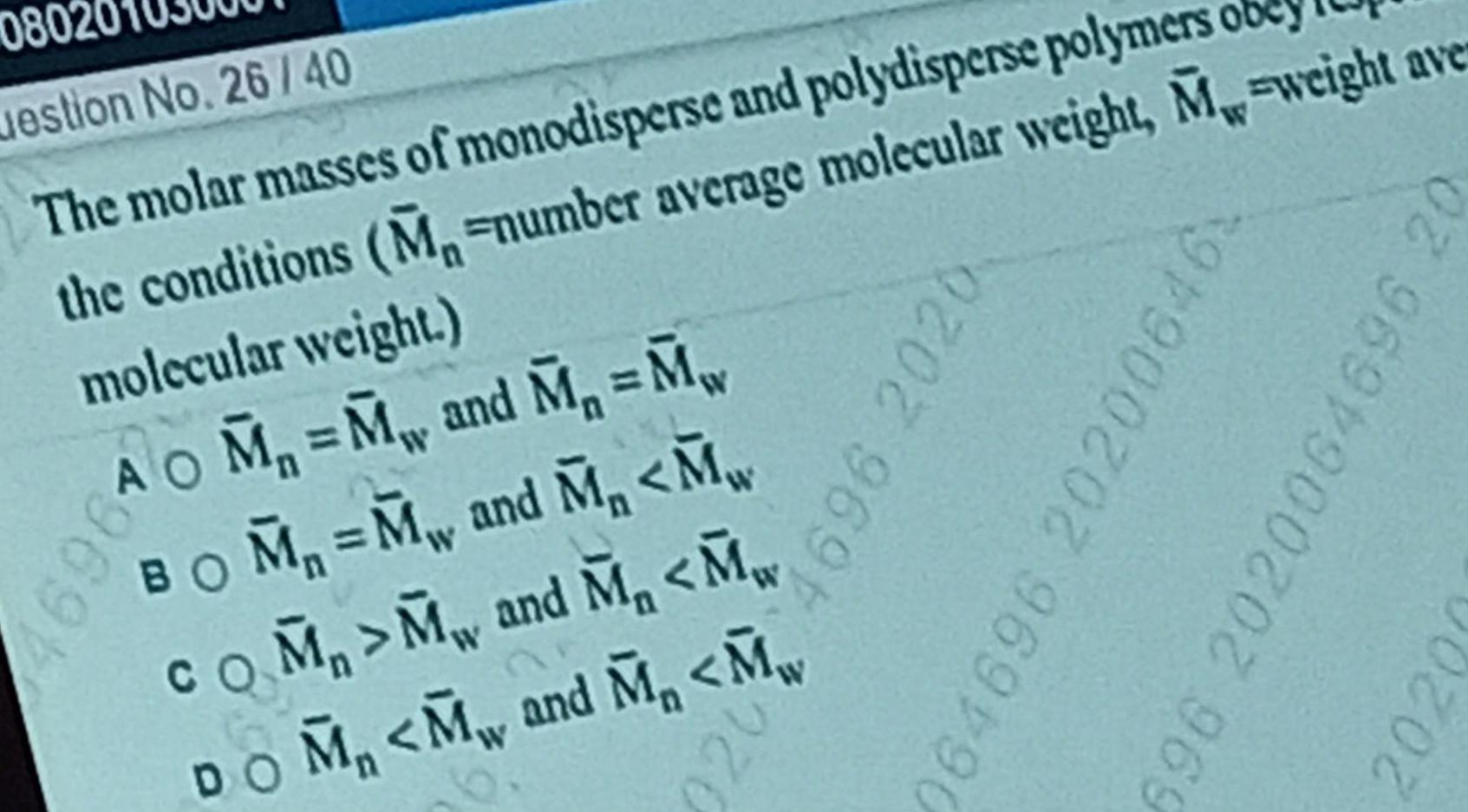
Physical Chemistry
Surface chemistry080 uestion No 26 40 The molar masses of monodisperse and polydisperse polymers obey the conditions M number average molecular weight M weight ave molecular weight 169610 M M and M M BOM M and M M COM M and M M DOM M and M M 9202 969 064696 202006 6 696 2020064696 20 2020

Physical Chemistry
Chemical BondingOn heating white colored powder stron gly in a boiling tube copper oxide black oxygen gas and a brown gas X is evolved a Identify the brown gas X evolved b Write balanced chemical equation o f the reaction c Identify the type of reaction
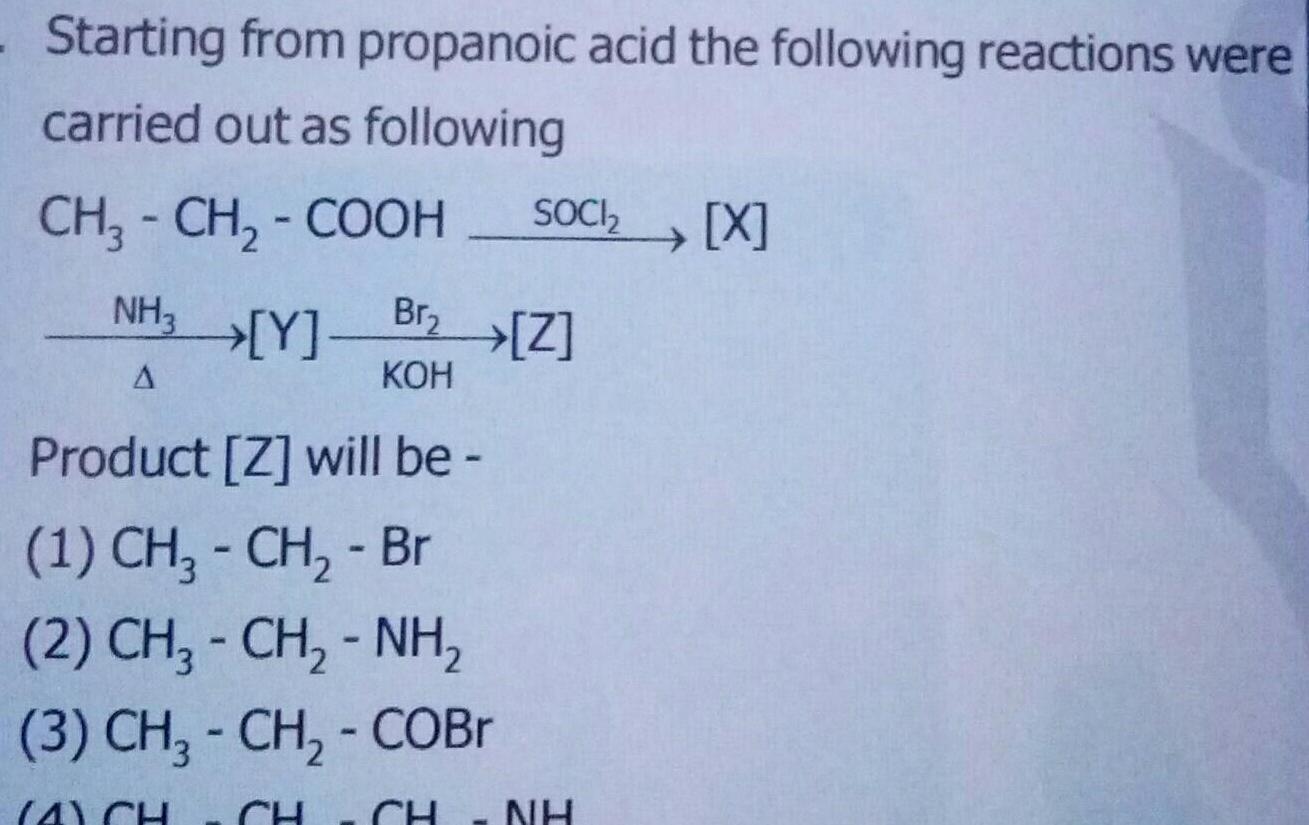
Physical Chemistry
GeneralStarting from propanoic acid the following reactions were carried out as following CH3 CH COOH NH3 Y A SOCI X Br Z KOH Product Z will be 1 CH CH Br 2 CH CH NH 3 CH CH COBr 4 CH CH NH

Physical Chemistry
General6 An electron is moving in 3rd orbit of Hydrogen atom The frequency of moving electron is 2 7 3 1014 rps 4 7 3 1010 rps 1 2 19 1014 rps 3 2 44 1014 rps hanical Modell

Physical Chemistry
Equilibrium4 9 The activation energy for the reaction 2 HI g H 1 g 2 is 209 5 kJ mol at 581K Calculate the fraction of molecules of reactants having energy equal to or greater than activation energy
Physical Chemistry
Equilibrium3 lon 85 100 mL of 0 02 M benzoic acid pK 4 20 is titrated using 0 02 M NaOH pH after 50 mL an 100 mL of NaOH have been added are a 3 50 7 b 4 2 7 c 4 2 8 1 d 4 2 8 25 86 What is the pH of a solution 10n

Physical Chemistry
Generalalculate the amount in gram of the sulphate ions olution MM 7200 IIT JEE Find the weight of sodium bromate and mola solution necessary to prepare 85 5 mL of 0 solution when the half cell reaction is ab BrO3 6H 6e Br 3H O4 Find the weight as well as molarity if the h reaction is 2BrO3 12H 10e Br 6H O tulo I uncin IIT JE A sample of hydrazine sulphate N H SO4 was d in 100 mL of water and 10 mL of this solution wa with excess of ferric chloride solution and wa complete the reaction Ferrous ion formed was e and it required 20 mL of M 50 potassium perm solution Estimate the amount of hydrazine sulp tog de Admi I of the solution