Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Chemical kineticsIf the rate constant of a reaction is 5 8 x 10 3 s1 what is the concentration after 100 seconds if the initial concentration was 5 0 M Hint What is the order of the reaction given the units of the rate constant Your Answer

Physical Chemistry
GeneralSubstitution of a purine base with a pyrimidine base or vice versa is called Transversion Transition

Physical Chemistry
Surface chemistryBredig s Arc method is suitable for making which of the following sol Gem stone O Gold sols Basic dye nr Gum arabic

Physical Chemistry
SolutionsAn element X of atomic mass 25 0 exists as X4 in benzene to the extent of 100 When 10 30 g of saturated solution of X in benzene is added to 20 0 g of benzene the depression in freezing point of the resulting solution is 0 51 K If K for benzene is 5 1 K kg mol the solubility of Xin 100 g of benzene will be

Physical Chemistry
Solutions21 a A Sample of ferrous oxide has actual formula Fe0 9301 00 In this sample what fraction of metal ions are Fe ions What type of non stiochiometric defect is present in this sample b A solution prepared by dissolving 1 25 g of oil of winter green methyl salicylate in 99 0 g of benzene has a boiling point of 80 31 C Determine the molar mass of this compound B P of pure benzene 80 10 C and K for benzene 2 53 C kg mol

Physical Chemistry
EquilibriumAcid lonization Equation H PO4 aq H aq HPO4 aq HPO4 aq H aq PO4 aq Acid lonization Constants Ka 6 3 10 8 Ka 4 5 x 10 13 pK 7 20 12 35 A buffer was prepared by mixing 200 0 mL of 0 500 M NaH PO4 and 50 00 mL of 0 400 M Na HPO4 1 Calculate the pH of the buffer Round off your answer until the second digit after the decimal point 2 Calculate the molar concentration of the buffer Observe rules on significant figures

Physical Chemistry
SolutionsThe freezing point of a mixture containing 1 60 g of naphthalene molar mass 128 g mol and 20 g of benzene molar mass 78 g mol is 2 8 C and that of pure benzene is 5 5 C The value of the molal freezing point depression constant of benzene is A 4 3 C kg mol 1 4 3 C mol kg 1 C B D mol 1 4 3 C g 5 1 C mol g 1

Physical Chemistry
GeneralAt industrial level which catalyst is not matched properly O Contact process for H SO4 V 05 O Haber s process for NH3 Fe O Ostwald s process for HNO3 MnO Cracking of hydrocarbon Zeolite

Physical Chemistry
Atomic Structure13 The mass of the nucleus is O A greater than the mass of the protons and neutrons that make up the nucleus OB equal to the mass of the protons and neutrons that make up the nucleus OC less than the mass of the protons and neutrons that make up the nucleus OD converted to energy
Physical Chemistry
Nuclear chemistry21 Nuclides produced by the decay of the parent nuclides Choose Rem Daughter Alpha Fusion Parent Positron Fission re nuclear radiation exposure re the dose of any type of ionizing radiation that factors iation has on human tissue

Physical Chemistry
Energetics17 In which of the enlisted cases Hess s law is no applicable a Determination of lattice energy b Determination of resonance energy c Determination of enthalpy of transformation o one allotropic form to another d Determination of entropy 76n passing 3 Ampere of electricity for 50 minutes 1 8 metal is deposited The equivalent mass o
Physical Chemistry
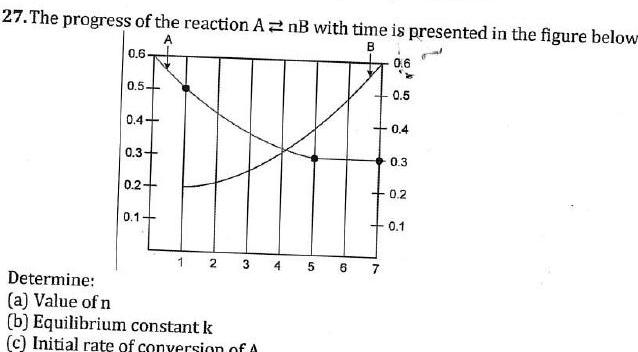
Equilibrium27 The progress of the reaction AnB with time is presented in the figure below A B 0 6 0 5 0 4 0 3 0 2 0 1 2 3 Determine a Value of n b Equilibrium constant k c Initial rate of conversion of A 4 5 6 0 6 7 0 5 0 4 0 3 0 2 0 1

Physical Chemistry
GeneralIn a ceremony the number of adults to the number of children who attended was 3 2 The number of men to the number of women was 2 3 There was an equal number of boys and girls There were 644 more women than girls a How many women were there b When some women left the ceremony the number of men and women remained became the same How many women left the ceremony

Physical Chemistry
SolutionsHydrochloric acid is prepared by bubbling hydrogen chloride gas through water What is the molarity of a solution prepared by dissolving 261 L of HCI g at 37 C and 9 99 atm in 5 83 L of water Add 273 to convert to Kelvin R 0 0821 Latm mol K Report your answer to three significant figures Your Answer

Physical Chemistry
GeneralOxidation and reduction process involves the transaction of electrons Loss of electrons is oxidation and the gain of electrons is reduction It is thus obvious that in a redox reaction the oxidant is reduced by accepting the electrons and the reductant is oxidised by losing electrons The reactions in which a species disproportionate into two oxidation states lower and higher are called disproportionation reactions In electrochemical cells redox reaction is involved i e oxidation takes place at anode and reduction at cathode Which one of the following is an example for disproportionation reaction Select an answer A MgO H O Mg OH 2 B C D P4 30H 3H O PH3 3H PO S O SO2 Zn CuSO4 ZnSO4 Cu

Physical Chemistry
GeneralWhen an aqueous solution was treated with AgNO3 a white precipitate was obtained which was soluble in NH OH The aqueous solution contained A Sulfate B Chloride C Acetate D Carbonate

Physical Chemistry
Solid stateAt room temperature sodium crystallizes in a body centred cubic lattice with a 4 24 The theoretical density of sodium At wt of Na 23 is A 1 002 g cm C 3 002 g cm 3 B 2 002 g cm 3 D None of these

Physical Chemistry
Equilibrium30 Which among the solutions given below will not show a change in pH on dilution I 0 1 M NH COOCH II 0 1 M NaCl III 0 1 M NH OH IV 0 01 M H SO4 A I and II B I II and IV C I and III D III and IV

Physical Chemistry
Chemical kinetics54 For a first order reaction A product the initial concentration of A is 0 1 M and after 40 minute iti becomes 0 025 M Calculate rate of reaction at reactant concentration of 0 01 M 1 3 47 10 M min 2 6 93 x 104 M min 3 3 47 x 10 5 M min 4 1 735 x 10 4 M min 16 23

Physical Chemistry
Chemical kineticsC4H8 6 02 4 CO 4H O Select the correct statement 213 Rate of disappearance of O2 is 3 of production of CO2 times rate Rate of disappearance of O is times rate of production of CO2 Rate of disappearance of C4H8 and O2 is same Rate of disappearance of O is less than rate of disappearance of C4H8

Physical Chemistry
General75 The rate constant of the reaction A B is 0 6 10 3 mole per second If the concentration of A is 5M then conentration of B after 20 minutes is 1 3 60 M 2 0 36 M 3 0 72 M 4 1 08 M

Physical Chemistry
GeneralMatch the following salts of Column 1 with the acids and bases which are used for their preparation with Column II List 1 1 KNO3 2 AgNO3 3 MgCl 4 NH4 2CO3 Answer 1 2 3 A A A List 2 A Nitric acid silver hydroxide B Hydrochloric acid Magnesium Hydroxide C Carbonic acid Ammonium hydroxide D Nitric acid Potassium hydroxide B B B C C C D D

Physical Chemistry
ElectrochemistryWhich one of the following does not hold good for S H E A The pressure of hydrogen gas is 1 5 atmosphere B The concentration of H in solution is 1M C The temperature is 298K D The surface of platinum electrode is coated with platinum black

Physical Chemistry
Generalsample of H atoms containing all the atoms in a particular excited state absorb radiations of a particular wave length by which the atoms get excited to other excited state When the atoms finally de excite to the ground state they emit radiations of 15 different wavelength Out of these 15 radiations 5 we wavelengths shorter than the absorbed radiation and 9 have wavelength longer than the absorbed radiation What is the initial excited state of ms
Physical Chemistry
Nuclear chemistry18 Particle with a positive charge and same mass as an electron Choose Rem Daughter Alpha Fusion Parent Positron Fission ich decay series ucleus splits into more stable nuclei of intermediate

Physical Chemistry
ElectrochemistryWhich of the following is are correct for potash alum B A alum Aq K SO 2q A1 eq B alum req Ne C alum eq D 1 4 eq q K Al 1 SO 3 4 eq akash eq alum 8 alum K A S0 Founda 00

Physical Chemistry
General34 Which of the following is correct for H ion A A possible trial wave function for the ion is C 1SA C 1SE B The coefficients of the trial wave function are not equal C A possible trial wave function is w C 1SA C 1SB D The 18 orbitals are not normalised

Physical Chemistry
Chemical kineticsMatch Column l with Column II Column l contains half life expression and Column Il contains corresponding order s rate constant expression a is the initial conc of reactant x is the concentration of product at any time t and k is the rate constant Column l Q1 t 2 Q2 t 2 Q3 t 2 Q4 t1 0 586 k 0 693 k a 2k ka Column II X k t A1 K A2 K K A3 K x A4 K tLa x A5 k a 2 303 log t a a x a x a

Physical Chemistry
Atomic Structureth According to Bohr s theory the energy in the n 2 176 10 18 x Z n remove an electron from the 2nd orbit of Li ion orbit is En 0 A 406 A What is the the longest wavelength of light needed to

Physical Chemistry
General7 The total number of neutrons present in 54 mL H 0 1 are P d a 3 NA b 30 NA pimots 9th c 24 NA are 8 Total number of electrons present in 48 g Mg in 48 g Mg 2 a 24 3 enigine d none of thes 180 as ni ang Pok

Physical Chemistry
Generalis denoted by the symbol v nu bar v 1 2 1 It is expressed in cm v Amplitude It is defined as the height of the crest or depth of the trough of a wave It is denoted by the letter a It determines the intensity of the radiation m Low energy Low frequency Long wavelength or m The arrangement of various types of electromagnetic radiations in the order of their increasing or decreasing wavelengths or frequencies is known as electromagnetic spectrum v 3x 107 cycle sec 2 cm 10 Wavelength TELEVISION WAVES RADIO WAVES MICROWAVES INFRARED VISIBLE Frequency ULTRAVIOLET 3 1021 11 10 X RAYS Y RAYS High energy High frequency Short wave length types of increasing or decreasing as electromagnetic spectrum The following order Electric field Magnetic field Fig 2 13 The electric a components of an elec Radio waves Television waves Microwav Ultraviole Each type of radiation is sn wavelength

Physical Chemistry
Surface chemistryYou have been given four charged sols Sol 1 Al O xH O Sol 2 CdS Sol Sol 3 TiO Sol Sol 4 Haemoglobin Consider the following statements S In electrophoresis the dispersed phase of only two of the given sols will move towards cathode S In electrophoresis the dispersion medium of only three of the given sols will move towards anode S3 Only three of given sols can be precipitated by K4 Fe CN 6 Which of the following options contain only the incorrect set of statement s

Physical Chemistry
Chemical kineticsFor the reaction A K B K C K 1 78 x 10 3 min 1 and k 5 8 x 10 5 min 1 when the initial concentration of A is 0 0174 molar at O C The time at which the concentration of B is maximum is X The value of in hr is to the nearest integer X 11 log 1 78 and log 5 8 are 0 2504 and 0 7634 respectively Answer 01 02 03 04 05 06 07 08 09 01 02 03 04 05 06 07

Physical Chemistry
Chemical kineticsThe decomposition of sulfuryl chloride SO2C12 is a first order process The rate constant for the decomposition at 660 K is 4 5 10 2 s 1 a If we begin with an initial SO2C12 pressure of 345 torr what is the pressure of this substance after 71 s torr b At what time will the pressure of SO2C12 decline to one sixth its initial value s By what factor will the pressure of sulfuryl chloride decrease after 8 half lives Note The answer wants this value 345 torr value final pressure

Physical Chemistry
GeneralAmong the given carbonates bicarbonates find the value of KHCO3 NaHCO3 CaCO3 Ca HCO3 2 K2CO3 and Mg HCO3 2 no of compounds almost insoluble in water x no of compound which liberate CO2 on reaction with HCl z no of compounds that easily undergo thermal decomposition y

Physical Chemistry
Chemical kineticsd Consider a first order reversible liquid reaction in the above batch reactor A R r kICA k CR with CAO 0 5 mol liter Cro 0 After 8 minutes conversion of A is 33 3 while equilib rium conversion is 66 7 Find the rate constants ky and kr Ans kj 2

Physical Chemistry
Electrochemistrylease explain The standard oxidation potential of zinc and silver in water at 298 Kare Zn s Zn 2e E 0 76 V 2 Ag s Ag 2e E 0 80 V IN SUPE Which of the following reactions actually take place NCERT 1983 84 KCET 2003 a Zn s 2Ag aq Zn aq 2 Ag s b c d Zn aq 24g s 24g aq Zn s Zn s Ag s Zn aq Ag aq Zn aq Ag aq Zn s Ag s C

Physical Chemistry
SolutionsColligative properties are very important properties of solution These are helpful in finding the molar mass of unknown compounds These properties are dependent on number of solute particles and not on its nature Consider the following curve 40 mmHg x X 1 12 W E mole fraction y 50 mmHg X 1 Read Less

Physical Chemistry
GeneralFill in the blanks 1 Silk is obtained from of silk moth 2 Silk fibre is formed of two strands of protein 3 Silk fibre is secreted by the of a silk moth of 4 Silkworm feeds on leaves 5 Breeding and management of silk moths for production of silk is called

Physical Chemistry
ElectrochemistryHow do we know which reaction is oxidation and which o ne is reduction Please explain 1 Which of the following is the cell reaction that occurs when the following half cells are combined E 0 54 V 1 2e 21 1 M Br 2e 2Br 1 M E 1 09 V a 2Br 1 Br 21 b 1 Br 21 2Br 21 Br 1 2Br d 21 2Br I Br Oxidation Reduction

Physical Chemistry
ElectrochemistryCopper reduces NO into into NO and NO depending upon the concentration of HNO3 in solution Assuming fixed Cu for reduction of NO into NO and NO by copper is same is 10 M The value of 2x is the nearest integer Given E Cu and PNo Pxo the HNO3 concentration at which the thermodynamic tende Rounded of Cu Cu 0 34 V ENO NO 0 96 V ENO NO RT 0 79 V and at 298 K 2 303 0 059

Physical Chemistry
Energetics5 For a free expansion of an ideal gas in an isolated chamber which of the following statements is true A Entropy of the system increases B Temperature of the system decreases C Internal energy of the system decreases D Positive work is done by the system

Physical Chemistry
Energetics28 The van der Waals coefficient a expressed in atm dm6 mol 2 for four different gases are He 0 0341 H 0 242 Kr 5 125 O 1 364 Based on the data given above the gas that will be expected to have the lowest critical temperature Te A He B H C Kr D 0

Physical Chemistry
Atomic StructureIf uncertainty in the measurements of position and velocity of electron are equal then minimum uncertainty in its velocity will be A B skipped C 1 2m 1 h am h 2 m QUES 1 6 Corr MY P S

Physical Chemistry
Gaseous and liquid states40 The P of real gases is less than the P of an ideal gas because of A Increase in number of collisions B Finite size of molecule C Increase in KE of molecules D Intermolecular forces D coate 148 Whic spon cell A B B F

Physical Chemistry
Solid stateCorrosion causes damage to carbon bridges iron railings ships etc Corrosion can be prevented by anti rust solution Galvanization is a method of protecting iron from rusting by coating them a thin layer of zinc Brass bronze steel etc are examples for alloys Two or more metals or a metal and a non metal combine and form a new substance is called alloy By making alloy we can increase the substance and Bleaching of coloured objects can be carried out by using chlorine Curfass

Physical Chemistry
GeneralQuestion 165 A certain oxide of iron contains 2 5 g of oxygen for every 7 0 g of iron if it is regarded as a mixture of FeO and Fe2O3 in the weight ratio x y atomic weight of iron 56 1 9 10 2 9 20 14 5 23

Physical Chemistry
EnergeticsWater is gaseous in 5L volume at 130 C and adiabatically expands to 10L volume at a constant pressure of 0 80 atm Find the temperature work internal energy enthalpy and temperature change in this time assuming that it is 5 mol of gas

Physical Chemistry
Solutionsa HCHO b CH3OH c C H5OH d C6H12O6 A 0 10 M solution of a monoprotic acid d 1 01 g cm is 5 dissociated What is the fre point of the solution The mol mass of the acid is 300 and K H O 1 86 C m

Physical Chemistry
Solutions2 51 A sample of clay was partially dried and then contained 50 silica and 7 water The original clay contained 12 water The percentage of silica in original sample is