Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
GeneralWhen 10 g of Paraffin wax C31H64 mw 436 84 is dissolved in water to make oil droplets depending on the radius r of the oil droplets Calculate the number and surface area of oil droplets d 0 82 g cm3 The volume is calculated from the mass the volume of one oil drop from the radius and the number of oil drops is calculated by dividing it After calculating the surface area of one oil drop from the radius multiply the number of oil drops to obtain the surface area of the oil drop Watch out for the unit

Physical Chemistry
Solutions3 What is the concentration of ethylene in units of grams per liter dissolved in water at 25 C when the C2H4 gas over the solution has a partial pressure of 0 247 atm k for C H4 at is 4 8x10 3 mol L atm

Physical Chemistry
EquilibriumAn analytical chemist is titrating 235 8 mL of a 0 6700 M solution of hydrazoic acid HN3 with a 0 2500M solution of KOH The pK of hydrazoic acid is 4 72 Calculate the pH of the acid solution after the chemist has added 749 0 mL of the KOH solution to it a Note for advanced students you may assume the final volume equals the initial volume of the solution plus the volume of KOH solution added Round your answer to 2 decimal places

Physical Chemistry
Solutions1 The boiling point elevation constant for benzene is 2 57 C m The boiling point of benzene is 81 C Determine the boiling point of solution formed when 10 g of C4H12 is dissolved in 20 g benzene 1 71 46 2 7 14 3 85 76 4 88 14

Physical Chemistry
Surface chemistryamount of adsorbent which of the following relations is not related to adsorption process CBSE PMT Pre 2011 X m pxT X f p at constant T m X f r at constant p m p f r at constant

Physical Chemistry
Generalde up of bases ic acid eaks down into and oxygen will not take Which of the following are made up of bases Select an answer A B C D Antacid tablet Soap Toothpaste All of the above

Physical Chemistry
Energetics3 4 4x X Enthalpy change on freezing of 1 0 mol of water at 40 C to ice at 0 C is AfusH 6 kJ mol at 0 C CP H O 1 75 J mol K NCERT Pg 172 1 2 3 4

Physical Chemistry
Electrochemistry78 The concentration of H and concentration of OH of a 0 1 aqueous solution of 2 ionised weak acid is ionic product of water 1 10 41 a 2 10 M and 5 10 2 M b 1 10 M and 3 10 1 M c 0 02 10 M and 5 10 1 M d 3 102 M and 4 10 3 M 1999 9 The solubility of a saturated solution of calcium fluoride is 2 x 10 moles per litra It 3H is a K 85 The ion Its ionic a 1 x c 1 x

Physical Chemistry
Chemical kineticsDecomposition 3A g 2B g 2C g follows first order kinetics Initially only A is present in the container Pressure developed after 20 minute and infinite time are 3 5 and 4 atm respectively Which of the following is true A 150 20 min C 199 64 3 min B D t75 40 min 185 70 min

Physical Chemistry
Gaseous and liquid statesT Select the correct statement s about given graph f 8 A Expansion takes place C Process is not isobaric A I C B Process is not isothermal D Process is not isochoric B D

Physical Chemistry
EquilibriumWhat is the equivalent mass of O2 in the following reaction H O O 2e 2 The amount of electricity which releases 2 0 g of gold from a gold salt is same as dissolves 0 967 g of copper anode during the electrolysis of copper sulphate soluti the oxidation number of gold in the gold ion At mass of Cu 63 5 Au 197 When a molten salt was electrolysed for 5 min with 9 65 A current 0 18 g of the deposited Calculate the Eq mass of metal og nohoubad During the electrolysis of a concentrated brine solution calculate the moles of produced by the passage of 4F electricity Calculate the cell potential in M if AG 965kl mol and n 1

Physical Chemistry
SolutionsDiatomic gaseous species A2 g decomposes into atomic A by first order Kinetics as A g 2A g An empty flask was filled with A2 g and N g at an initial pressure of 800 mm of Hg at 600 K and sealed After a very long time gases in the flask developed 1400 mm of Hg If half life for the decomposition process is 2 h what was the pressure in the flask after 4 h Assume N 2 to be inert gas

Physical Chemistry
Gaseous and liquid statesa P P c P P b P P d All of these 22 Two flasks A and B of 500 mL each are respectively filled with O and SO2 at 300 K and 1 at pressure The flasks will contain a the same number of atoms b the same number of molecules c more number of moles of molecules in flask A as compared to flask B d the same amount of gases ylem d d ALEBO C

Physical Chemistry
Atomic StructureSummary of Dual Nature of Radiation The various phenomena concerning radiation can be divided into three parts i The phenomena such as interference diffraction polarisation etc in which there is interaction between radiation and radiation can be explained on the basis of wave nature of radiation The phenomena such as black body radiation photoelectric effect etc in which there is interaction between radiation and matter can be explained on the basis of quantum theory of radiation i e particle nature iii The phenomena such as rectilinear propagation reflection refraction etc in which neither there is interaction between radiation and radiation nor between radiation and matter can be explained with either of the concepts i e either by wave theory or by photon theory 2 x 10 5 6 63 10 19 1 10 5 1 6 63 x 10 34 4 The number of photons of light having waveleng nm which can provide 1 J energy is nearly a 107 photons c 5 x 10 7 photons b 5 x 10 8 photons d 5 x 107 photons Ans c Hint E hc n EX hc 1x 100 x 10 6 626 x 10 34 3 10 5 10 7 5 The atomic transition gives rise to the radiat frequency 10 MHz The change in energy per r atoms taking place would be a 3 99 x 10 6 J c 6 62 x 10 24 J Ans b b 3 99 J d 6 62 x 10 30 J

Physical Chemistry
Chemical kineticsIn the prior video we learned that the ratio of rate constants KA KB e AE RT where R 1 9872 cal mol K 1 Select the best answer for the product ratio A C at the end of the reaction if the the activation energy for B going to A is 4 2 Kcal mol higher than the activation energy for B going to C at room temperature 22 C 295 15 K and given the fact that the reactions of B going to A and B going C are both irreversible a A C 1 015 b A C 0 985 c A C 0 000776 d A C 1 288 A B KA KB e AE RT

Physical Chemistry
GeneralQ 58 The weight of CO is required to form Re CO 10 will be g from 2 50 g of Re O according to given reaction Re O CO Re CO 10 CO2 Atomic weight of Re 186 2 C 12 and 16 WA

Physical Chemistry
Generala CH3COONH4 have greater deg outs solution CORTE RI b Anions which are weaker base than OH do not hydrolyse c The CH3COO have greater degree of hydrolysis in comparision salt solution have equal conc d So hydrolyses but HSO does not undergo hydrolysis 0 01M NH4Cl aq solution at 25 C has a Cl aq 10 M i c DOH 7 SHO oldgifsen HM HMM b NH aq 10 2M d H 10 7 Mond

Physical Chemistry
Energetics6 If enthalpy of combustion of carbon to CO2 is 400 kJ mol 1 then how much heat will be released upon formation of 8 8 g CO2 from carbon and dioxygen gas NCERT Pg 176 1 40 kJ 2 80 kJ 3 400 kJ 4 800 kJ 7 A reaction is non temperatures when spontaneous at all NCERT Pg 186 O and A S 0

Physical Chemistry
General92 In liquid gas equilibrium the pressure of vapours above the liquid is constant at a constant temperature b low temperature c high temperature d none of these 1995 93 Which one of the following is most soluble a Bi S3 Kp 1 x 10 70 sp 6 x 10 51

Physical Chemistry
GeneralAn element X on exposure to moist air turns reddish brown and a new compound Y is formed The substance X and Y are Select an answer A B C D X Fe Y Fe O3 X Ag Y Ag S X Cu Y CuO X Al Y Al2O3

Physical Chemistry
GeneralNitrobenzene on reaction with conc HNO3 H2SO4 at 80 100 C forms which one of the following products 0 3 orrect Answer 3 our Answer 4 tatus incorrect 1 2 4 Trinitrobenzene 1 2 Dinitrobenzene 1 3 Dinitrobenzene 1 4 Dinitrobenzene Add to Bookmark List
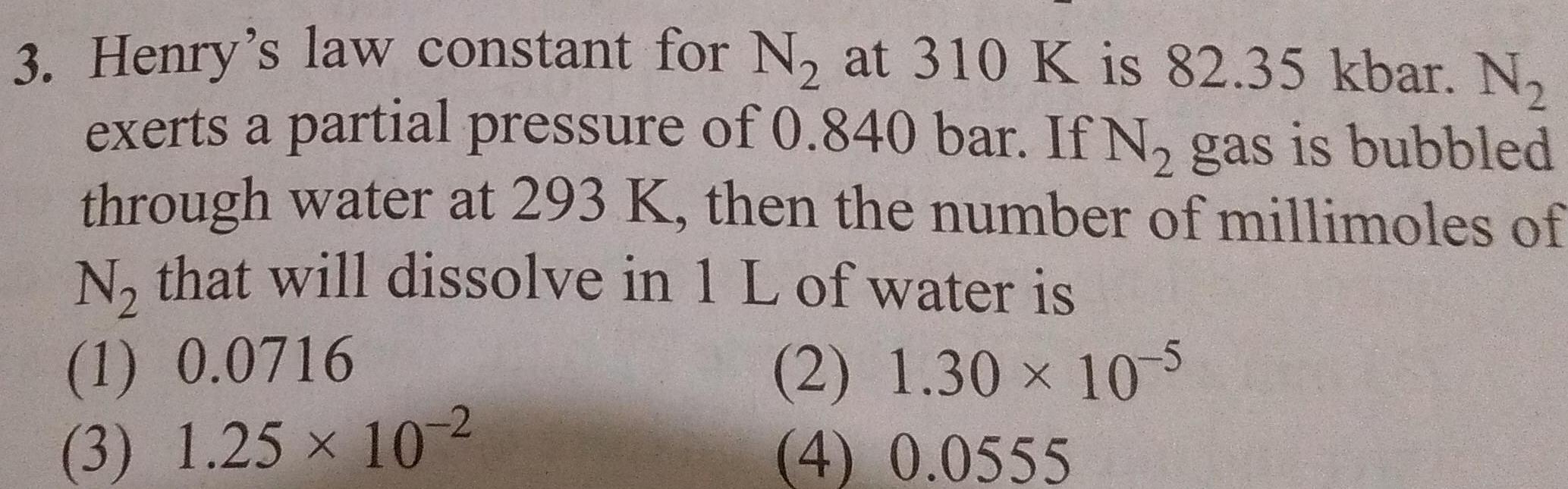
Physical Chemistry
Solutions3 Henry s law constant for N at 310 K is 82 35 kbar N exerts a partial pressure of 0 840 bar If N gas is bubbled through water at 293 K then the number of millimoles of N that will dissolve in 1 L of water is 2 1 30 10 5 4 0 0555 1 0 0716 3 1 25 10 2

Physical Chemistry
Chemical Bonding54 82 litres of carbon dioxide are produced at a pressure of 154 atm by the action of acid on a metal carbonate The work done by the gas in calories in pushing back the atmosphere is R 0 082 litre atm deg mot 1 1000 3 1640 4 2000 4 137 7 kJ 1 atm 82 andrests the rahat at the groe av befi i R 0 082 litre atm deg mot 1 1000 2 820 3 1640 4 2000 3 45 9 k

Physical Chemistry
Atomic StructureWhich of the following statement s is are incorrect Options 1 The electronic configuration of Cr is Ar 3 d5 4 s Atomic Number of Cr 24 The magnetic quantum number may have a negative value 2 3 In Ruthenium atom 20 electrons have a spin of one type and 24 of the opposite type Atomic Number of Ru 44 A The owvidatic regen in HN in

Physical Chemistry
Equilibrium7 Which one of the following information can be obtained on the basis of Le Chatelier principle a Dissociation constant of a weak acid b Entropy change in a reaction c Equilibrium constant of a chemical reaction d Shift in equilibrium position on changing value of a constraint 1992 98 Aqueous solution of acetic acid contains a CH3COO and H

Physical Chemistry
GeneralThe set representing the incorrect order Options 1 Si P C N 2 F CI Br 3 Na Mg Al Si 4 O F N C Order of electro negativity Order of electron affinity Order of first ionization potential Order of second ionization potential

Physical Chemistry
General2 46 First order reaction is given X Y where X and Y both are optically active compounds with specific rotation 80 mole and 10 mo respectively Initial rotation of pure sample of X was 20 and solution becomes inactive after 22 min the rate constant in min of given reaction is Given In 2 0 7 and In3 1 1 B 0 05 A 0 01 C 0 1 D 0 5 merts
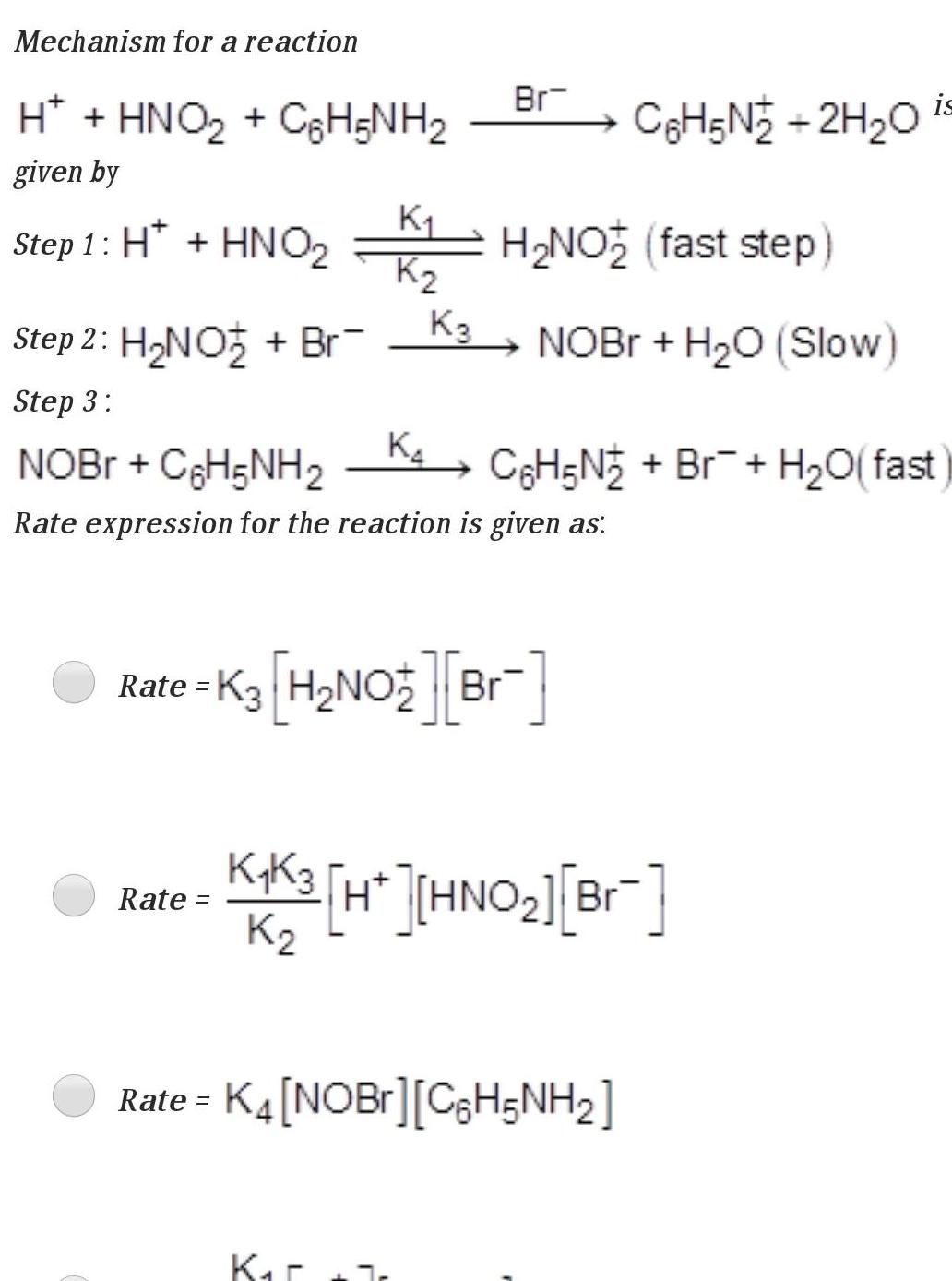
Physical Chemistry
Chemical kineticsMechanism for a reaction H HNOz CoH NH given by Step 1 H HNO2 Step 2 H NO Br Step 3 K K Rate K3 Br K NOBr C6H5NH Rate expression for the reaction is given as Kar H NO fast step NOBr H O Slow Rate K3 H NO Br is C6H5N 2H O C6H5N Br H O fast K4K3 H HNO Br K2 Rate K4 NOBr C H5NH 2

Physical Chemistry
EnergeticsGases AHJ mole P A g B g C g Q C g E g D g F g B g C g R A g B g C g D g E g F g 30 20 40 60 80 100 1 2 3 Spontaneous at high temperatur Spontaneous at all temperature Spontaneous at low temperature

Physical Chemistry
GeneralWhen lead nitrate is heated it breaks down into lead monoxide nitrogen dioxide and oxygen 2 Pb NO3 2 2PbO 4NO2 O The reaction is an example of Select an answer A Combination reaction B Decomposition reaction C Double decomposition reaction Displacement reaction

Physical Chemistry
GeneralThe number of moles of atoms in 1030 g of Rhodium is x times that of moles of Carbon in 60 g Carbon y times that of moles of Magnesium in 48 g of Magnesium and z times that of moles of Rhenium in 465 5 g of Rhenium Then find the value of x y z

Physical Chemistry
EnergeticsIn an isothermal process 3 moles of an ideal gas expands from pressure P1 to P2 against constant opposing pressure of P2 Calcu following Note Here P is constant but P is not ext a Change in enthalpy b Work done c Change in Internal energy d Heat transferred

Physical Chemistry
Surface chemistryTo make negative sols Agl which of the following mixin is favoured 10 ml 0 1 M AgNO3 10 ml 0 1 M KI 10 ml 0 1 M AgNO3 12 ml 0 1 M KI 12 ml 0 1 M AgNO3 8 ml 0 1 M KI hr mir All of these

Physical Chemistry
Chemical kineticsConsider the following reaction A g B s C g The following data is given for the decomposition of A into B and C at 300 K and the reaction follows first order kinetics Time min 205000 Vol of C mL 1025160 It is given that vapour pressure of B s is negligible In16 2 77 In15 2 70 Identify the correct statement 0 Pate constant of reaction in 0 0035s Rate of formation of Band C will be equal Volume of C Volume of A at time 100 min

Physical Chemistry
ElectrochemistryAgCl Ag can be most easily coagulated by least concentration of O Br O co O PO O Fe CN 4

Physical Chemistry
Chemical Bonding90 Which statement in correct O All minerals are ores Mark for Review O A mineral cannot be an ore An ore cannot be a mineral O All ores are minerals

Physical Chemistry
Chemical kineticsthe paragraph carefully and answer the questions given below it 4A The rate constant of a reaction following first order kinetics can be determined with th help of titrations also For example if the following decomposition is taking plac 2B aq 3C following first order kinetics if all A B and C are oxidisable wit n factors n n and no respectively During titration with an oxidizing agent Let at t 0 volume used of reagent be V Let at t t volume used of reagent be V Let at t volume used of reagent be V The rate constant for above reaction if only B and C are participating in the titrations AV 2 303 V B K A K t V C K 2 303 t 2 303 t log log V V V D K 2 303 t log 4V V log V V

Physical Chemistry
Chemical kineticsRate constant K varies with temperature as given by equation 1000 K log 0 K min T Consider the following about this equation 1 Ea is 4 606 Kcal II Pre exponential factor is 10 III Variation of log K with is linear 1 T Which of the following statements are correct I II II III I II III I III

Physical Chemistry
Electrochemistry6 Mark the correct choice of electrolytes represented in the graph Am S cm mol YO A B C1 2 mol L 1 1 2 a A NH OH B NaCl b A NH OH B NH CI c A CH COOH B CH3COONa 1 NILI

Physical Chemistry
Atomic StructureA H like species is in their excited state A and absorbs a photon of energy 3 868 eV and get excited to a new state B The electron from B on returning to a lower orbit can give a maximum of ten different emissions Some of the radiations have energies greater than it and some equal to 3 868 eV Exactly 2 radiations have energies less than 3 868 eV Determine the orbit numbers of states A and B and also identify the species

Physical Chemistry
GeneralTollowing is Options 1 the first ionization potential of Al is less than the first ionization potential of Mg 2 the second ionization potential of Mg is greater than the second ionization potential of Na 3 T Bi 3 and Pb 2 are stable due to inert pair effect 4 size of Al is larger than Ga

Physical Chemistry
Equilibrium91 The solubility of AgCl will be minimum in a 0 01 M CaCl c 0 001 M AgNO3 b pure water d 0 01 M NaCl 1995 92 In liquid gas equilibrium the pressure of

Physical Chemistry
Chemical BondingWhich among the following is froth stabiliser in froth floatation process Xanthates Aniline NaCN Pine oil

Physical Chemistry
SolutionsWhen a gas is bubbled through water at 298 K a very dilute solution of gas is obtained Henry s law constant for the gas is 100 kbar If gas exerts a pressure of 1 bar the number of moles of gas dissolved in 1 litre of water is a 0 555 c 55 55 x 10 b 55 55 x 10 5 al d 5 55 x 10 500

Physical Chemistry
Chemical kineticsMark for Review Which of the following is not true regarding catalyst O Catalyst does not change equilibrium constant value O Coenzyme increases the catalytic activity of enzyme All of these hr r O Catalyst can also catalyse non spontaneous reaction

Physical Chemistry
ElectrochemistryWhich of the following does not behave as emulsifying agent O Lampblack O Gold sols O Synthetic soaps Gums

Physical Chemistry
Surface chemistryets are filled with c hydrogen gas b carbon dioxide d nitrogen Identify incorrect statement from the following a During rainy season the power supply to our home from the electric pole will be interrupted due formation of metallic oxide layer on the electric wire b Vitamin E and Vitamin C are food preservatives prevent the food from spoilage c Food contianing fats and oils are stored in air tight containers or filled with Nitrogen gas d Rancidity is a reduction reaction 81

Physical Chemistry
GeneralWhich of the given disorders is inborn error of metabolism Phenylketonuria Cystic fibrosis Haemophilia Turner s syndrome

Physical Chemistry
SolutionsWhich of the following is an incorrect property of colloids O Size of particle 1 to 1000 nm Values of colligative properties are high for colloidal solution then for true solution O Heterogenous and settle due to centrifuge Shows Brownian movement and Tyndall effect

Physical Chemistry
EquilibriumFor the dissociation A B3 g 2AB g B g 2 If M Molecular mass of A B3 g D Vapour density of equilibrium mixture po Initial pressure of A B3 g then identify the CORRECT statement s A Equilibrium pressure can be expressed as B Equilibrium pressure can be expressed as 2P D M P M 2D M 2D 3D C Degree of dissociation of A B3 g can be expressed as D Increase in temperature will increase the magnitude of D