Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Equilibriumml of 39 0 01 M HA aq is 2 dissociated OH of solution is a 2x 10 4 b 10 8 c 5 x 10 11 d 5 10 2 40 If degree of dissociation is 0 01 of decimolar solution of weak acid HA then pK of acid is

Physical Chemistry
ElectrochemistryDuring electrolysis of aq CH3COONa solution current of 965 A is passed for 10 second using Pt electrodes select the correct statement s Take 1 F 96500 C mol 1 12 L of H gas at STP is evolved at cathode C H6 gas evolved at anode If the hydrocarbon gas evolved at anode is burnt with excess of O the moles of CO gas evolved is 0 2 During electrolysis total moles of gases evolved is 0 2

Physical Chemistry
Gaseous and liquid statesAt 0 C when the density of a certain oxide of a gas at 3 bar is same as that of dioxygen at 5 bar the molecular mass of the oxide is Options 53 3 45 5 19 2 20 7 Solution

Physical Chemistry
SolutionsState and explain the van t Hoff s factor i A sample of camphor K 40 melts at 176 C A solution of 0 0205gm of a hydrocarbon in 0 261gm camphor melts at 156 C The hydrocarbon contains 92 3 carbon Determine the molecular formula of the hydrocarbon 2 2

Physical Chemistry
Chemical kineticsThe rate constants of two reactions at two different temperatures are 1 8 10 5 mol Ls and 2 4 x 10 5 mol 1 Ls 1 The order of the reaction can be second order data insufficient zero order first order

Physical Chemistry
GeneralWrite down the atomic valency of the fir st 90 elements Write one by one valency of these elem ents Don t write any method only right valency Don t reject this question as my mentor

Physical Chemistry
Energetics149 The combustion of benzene 1 gives CO g and H O I Given that heat of combustion of benzene at constant volume is 3263 9 kJ mol at 25 C heat of combustion in kJ mol of benzene at constant pressure will be R 8 314 JK mol A 3267 6 C 452 46 B 4152 6 D 3260 15

Physical Chemistry
SolutionsIdentify the incorrect statement s Assume no change in volume of solution On adding HCI to NaOH aqueous solution the boiling point of the solution decreases initially and then gradually increases On adding NaOH to Ca OH 2 aqueous solution the freezing point increases initially On adding HCI to NaOH aqueous solution the relative lowering in vapour pressure increases initially then decreases On adding water to a solution containing 0 1 M NaOH and 0 1 M HCl boiling point of solution will decrease

Physical Chemistry
Atomic Structureed option is incorrect o 0 In case if no response is typed selected The energy needed to excite an electron from ground state to the 1st 2nd and 3rd excited states are 10 eV 15eV and 17 eV If 16 eV energy is provided to the electron and if this energy does not match with the energy difference between any two levels the electron O will go to 2nd excited state and 1 eV energy will be converted to its K E O Can go to the 1st excited state and 6eV energy will be converted to K E O will not excite at all O will remain in between 2nd and 3rd excited state

Physical Chemistry
EquilibriumThe reaction A s 2B g is in equilibrium at 4 atm and 27 C If the volume of system is increased at constant temperature then at new equilibrium relative to initial equilibrium A Moles of A s decreases B Moles of B g increases C Molar concentration of A s decreases D Molar concentration of B g decreases

Physical Chemistry
EquilibriumAt 25 C in one saturated solution of silver oxalate K 1 6x10 0 1 moles of Na C O a added If 1 60 Scm mol 140Scm mol 2 1 2 Na 50Scm mol 1 then select correct co statement A B C Conductance of given solution is nearly 24x10 S Conductivity of given solution is nearly 24 10 Scm If cr is 50 Scm mol then 120Scm mol AgC1 Solubility of Ag C 0 in presence of Na C O is

Physical Chemistry
Equilibrium38 A weak base MOH of 0 1 N concentration shows a pH value of 9 What is the percentage degree of dissociation of the acid b 0 001 a 0 01 c 0 1 d 0 02 39 0 01 M HA aq is 2 dissociated OH of solution is

Physical Chemistry
Chemical BondingWhich of the following statements about hydrogen bonds is true Select ALL that apply Hydrogen bonds can occur between water molecules Hydrogen bonds occur between hydrogen atoms bound to an electronegative atom and another electronegative atom Hydrogen bonds are one of the strongest types of chemical bonds Hydrogen bonds can occur between macromolecules like proteins Hydrogen bonds are type of non polar covalent bond

Physical Chemistry
EquilibriumCalculate the carbonate ion concentration CO3 2 in a 0 10 M solution of the weak acid carbonic acid H CO3 The dissociation constants of carbonic acid are Ka 4 5 x 10 7 and Ka 4 7 x 10 11 Question Type Single Correct Type 14 7 10 11 M 2 1 0 107 M 3 4 5 x 10 7 M

Physical Chemistry
Generalch of the following statement is correct a Molecular mass of dry air is less than moist air b Molecular mass of dry air is greater than moist air c Molecular mass of dry air is equal to moist air d Molecular mass of dry air may be greater or less than moist air

Physical Chemistry
General2 Using the data given below find out the strongest reducing agent Ec cr 1 36 V 2 Cr O Cr3 1 33 V 1 33V E 2 A Mn MnO4 Mn 3 C Cr 1 51V 1051V 1 36V 1 36V1 3 ECr Cr 0 74 V LB Cr 0 74V

Physical Chemistry
SolutionsFor the following reaction the equilibrium constant K at 298 K is 16 x 10 7 C 2 Fe aq S aq FeS s 2 When equal volumes of 0 06 M Fe aq and T 0 2 M S aq solutions are mixed the equilibrium concentration of Fe aq is found by Y x 10 7 M The value of Y is IDE

Physical Chemistry
Chemical BondingThe oxidation state of O in H O H O2 and KO2 respectivel are 2 1 1 2 2 2 2 1 1 1 2 1 2 12 2 1 1

Physical Chemistry
Atomic Structure34 The second order Bragg diffraction of X rays with 1 00 from a set of parallel planes in a metal occurs at an angle 60 The distance between the scattering planes in the crystal is a 2 00 b 1 00 c 0 575 d 1 15 1998 35 The edge length of face centred unit cubic
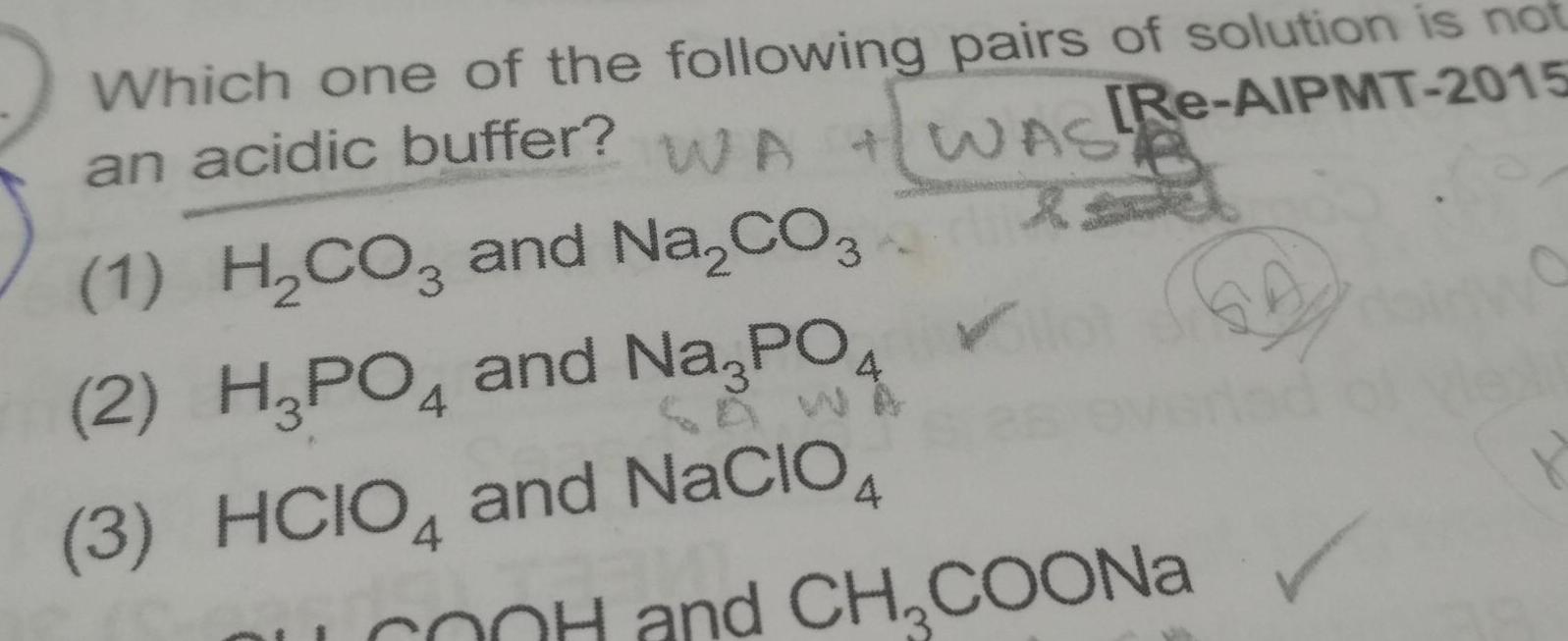
Physical Chemistry
Atomic Structurean acidic buffer WA Which one of the following pairs of solution is not Re AIPMT 2015 WASA 1 H CO3 and Na CO3 2 H3PO4 and Na3PO 3 HCIO4 and NACIO4 4 OOH and CH COONa

Physical Chemistry
Gaseous and liquid statesVander waal s gas equation may be expressed as B C Z 1 V m V m Where V molar valume of gas If B 0 105 L mol and C 4 x 10 4L mol 2 at 127 value of Vander waal constant a in atm L mol 2 is R 0 08 L atm K mol

Physical Chemistry
GeneralII Applicatio 1 Balance the following chem a Calcium hydroxide s Nitric acid b Magnesium s lodine s Magnesium lodide s 2 Write the following chemical reactions including the physical states of the substa balance chemical equations AS a Sodium Hydroxide reacts with Hydrochloric acid to form Sodium Chloride b Barium Chloride reacts with liquid Sodium Sulphate to leave Barium Su precipitate and also form liquid Sodium Chloride Higher Order Thinking Questions 2 moles of Zinc reacts with a cupric choloride solution containing Formula units of CuCl Calculate the moles of copper obtained AS ZnCl Cu s Zn CUCI aq 2 aq mole of propane C H on combustion at STP gives A kilo joules of aliculate the heat libarated when 2 4 ltrs of propane on combustion a liculate the mass and volume of oxygen required at STP to conv

Physical Chemistry
Solutionsskipp Moles of CO obtained on complete oxidation of 22 g of propane in presence of excess of oxygen is Options 1 5 2 2 5 0 5
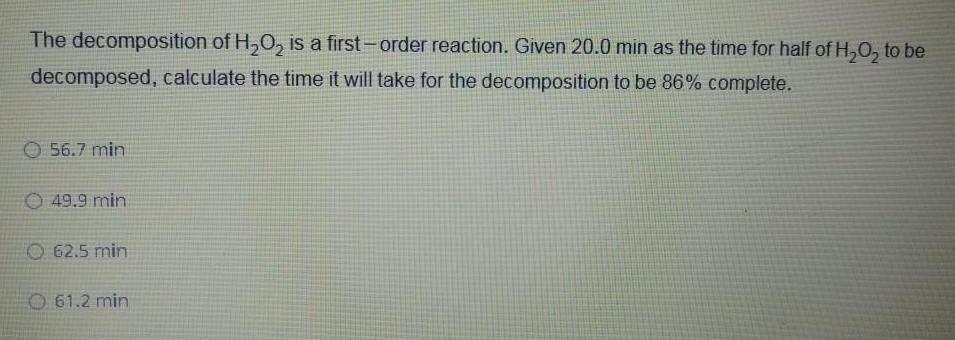
Physical Chemistry
Chemical kineticsThe decomposition of H O is a first order reaction Given 20 0 min as the time for half of H O to be decomposed calculate the time it will take for the decomposition to be 86 complete 56 7 min 49 9 min 62 5 min O 61 2 min

Physical Chemistry
GeneralA solution of 5 gm of Haemoglobin molecular weight 64000 in 100 cc of solution shows a temperature raise of 0 031 C for complete oxygenation Each mole of haemoglobin binds 4 mole of oxygen If the heat capacity of the solution is 4 18 k cm calculate AH per gm mole of oxygen bond

Physical Chemistry
GeneralThe following reaction NH4Cl s NH3 g HCl g have 2 phases 2 constituents 3 components 3 phases 3 constituents 3 components Ob OC O d 2 phases 3 constituents 3 components 2 phases 3 constituents 1 component

Physical Chemistry
GeneralA liquid solvent is added to a flask containing an insoluble solid The total volume of the solid and liquid together is 80 0 mL The liquid solvent has a mass of 28 9 g and a density of 0 865 g mL Determine the mass of the solid given its density is 1 75 g mL

Physical Chemistry
Solutions35 The nature of graph for effect of concentration on rate of reaction between 0 1 M Na 2S 20 3and 1 M HCI is a straight line with a decreasing slope b increasing slope c increasing slope intersecting to y axis d decreasing slope intersecting to x axis 1 pc

Physical Chemistry
SolutionsConsider the following diagram P J Solution SPM P 2 Solvent Container 1 Container 2 SPM is the semi permeable membrane P and P are the pressure applied Identify the incorrect statement If P P then liquid will flow from container 2 to container 1 To stop the osmosis P must be equal to osmotic pressure To carry out osmosis P must be lesser than P2 If P is greater than P then reverse osmosis will occur 2

Physical Chemistry
GeneralIn the Kjeldahl s method for estimation of nitrogen present in an organic compound ammonia evolved from 0 8 g of organic compound neutralizes 20 ml of 0 8 M H SO4 The percentage of nitrogen in the organic compound is 28 56 O 33

Physical Chemistry
Solid stateelement X has two crystalline forms P CsCl type and Q ZnS type zinc blende in which both the positions of cations and anions are occupie the atoms of element X dentify the correct statement s Ratio of mass of unit cell of P type and of Q type crystalline form is 4 The ratio of packing fraction of P type and Q type crystalline form is 2 approximately The ratio of packing fraction of P type and Q type crystalline form is 4 approximately The ratio of density of P type and Q type crystalline form is 2 approximately

Physical Chemistry
Solutions0 005 mol of Kl react with excess of CuSO and PRE 4 form 12 The 1 requires x L of 1 M Na S O 3 2 solution The total moles of sulphur atom in zero oxidation state in product are y mol The value of 1000 2y 2x is 14

Physical Chemistry
EquilibriumCalculate CH3COO concentration in aqueous solution of 0 01 M acetic acid containing 0 1 M HCI Given K for CH3COOH 1 8 x 10 1 O 1 8 x 10 5 mol L 1 O 1 8 x 10 6 mol L 1 O 3 6 x 10 3 mol L 1 O 5 4 x 10 5 mol L 1

Physical Chemistry
Chemical kineticsThe following data were obtained during the first order thermal Exam decomposition of N O5 g at constant volume 2 2N O5 g 2N2O4 g O g S No T 1 2 Time s 0 100 Calculate the rate constant Total Pressure atm 0 5 0 512

Physical Chemistry
GeneralConsider the reaction represented by the equation 25O g O2 g 2SO3 g For the system at chemical equilibrium which of the following explains what happens after the addition of sulfur trioxide assume constant temperature The amount of SO3 g increases and the value for K stays the same The amount of SO3 g increases and the value for K increases O The amount of SO3 g decreases and the value for K stays the same O The amount of SO3 g decreases and the value for K increases O The amount of SO3 g stays the same and the value for K decreases

Physical Chemistry
Solutions100 ml solution of FeC O and FeSO is completely oxidised by 60 ml of 0 02 M KMnO in acidio medium The resulting solution is then reduced by Zn and dil HCL The reduced solution is again oxidised completely by 40 ml of 0 02 M KMnO4 What is molarity of FeC 0 in solution

Physical Chemistry
Chemical Bondingfrom the given data select correct statements A at 27 degree C C can act as reducing agent for BO B at 27 degree C C can act as reducing agent for AO C at 27 degree C A can act as reducing agent for BO D at 27 degree C A can act as reducing agent for AO PLZ PROVIDE WITH STEP BY STEP SOLUTION ALTER In metallurgy concentrated oxide ores arverted into metal with the help of suitable reducing agent Data is given below for some reactions Reactions 1 2A s O g 2AO g II 2B s O2 g 2BO g III 2C s O g 2CO g Reaction 1 AHO kJ mol AS J mol K 100 11 70 60 280 210 160

Physical Chemistry
Equilibrium15 A hand book states that the solubility of RNH g in water at 1 atm and 0 C is 22 41 volumes of RNH g per volume of water pK of RNH 4 Find the max pOH that can be attained by dissolving RNH in water a 1 b 2 c 4 d 6

Physical Chemistry
Gaseous and liquid states80 ml of O takes 2 minute to pass through the hole what volume of SO will pass through hole in 3 min S 32 O 16 2 120 2 3 120 2 12 S 4 12 2 Vander waal constant a accounts for

Physical Chemistry
General53 Which of the following substituted carboxylic acid has the highest K value 1 CH CH CH COOH Cl 3 6 2 CH CH CH COOH 3 CH CH CH COOH 1 4 CH CH CH COOH

Physical Chemistry
Atomic StructureP Oa The state of an ideal gas is changed in a closed path 1 2 3 4 1 Which of the following is true about work done on the gas 1 Work 1 2 W 0 Work 1 2 Ob w 0 Work 1 2 W 0 13 dW 0 4 Work 2 3 W 0 Work 2 3 W 0 Work 1 2 Work 2 3 W 0 V Work 2 3 W 0 Work 3 4 W 0 Work 3 4 W 0 rk 3 W 0 Work 3 4 W 0 Work 4 1 W 0 Work 4 1 W 0 Work 4 1 W 0 Work 4 1 W 0

Physical Chemistry
GeneralIf the molecularity of an elementary reaction is two whereas its order is one then the reaction is known as O Second order reaction Pseudo first order reaction O Pseudo unimolecular reaction Both 2 and 3 SEUDO FIRST ORDER REACTION For an elementary chemical reaction the order is same as molecularity In several reactions the order is different from molecularity This is particularly the case when one of the reactants is present in large excess The molecularity of acidic hydrolysis of sucrose and ester is two where as its order is one So such reactions coudo first order reaction

Physical Chemistry
Solid statemaken the type of crystals Column 11 Column 01 KCI type crystal 02 ZnS Zinc blende type crystal with the appropriate relation involving edge lengin a radius of cation 14 and radius of anion r given in Column A1 5 4r A2 5 28 A3 2 1 03 CaCl type crystal 04 A B type crystal in which anion B forms simple cubic unit cell and cation A occupies all 44 2 G

Physical Chemistry
ElectrochemistryWhen excess of an electrolyte is electrolysed using a direct current source the mass of substance deposited at cathode depends on assume that the same charge is used in each case and same substance is deposited in each case A Temperature B Molten or aqueous electrolyte C Electrochemical equivalent of that substance D Solvent 001100110 0 n nouoou

Physical Chemistry
Equilibriumligh levels of ozone 03 make rubber deteriorate green plants turn brown and cause people to have ifficulty breathing Calculate AG at 298 K for the formation of 1 mole of Os from O in urban smog here 0 0 210 atm and 03 5 00 x 107 atm kJ mol

Physical Chemistry
Solid stateGraphite is an example of Question Type Single Correct Type 1 lonic solid 2 3 Covalent solid Vander Waal s crystal Metallic crystal

Physical Chemistry
Chemical kineticsYou experimentally determine the rate of reaction at different temperatures while keeping the init concentrations of the reactants constant What relationship would you expect to find between temperature and rate O As temperature increases rate decreases O As temperature increases rate increases The relationship depends how many reactants are present Temperature has no effect on the rate of a chemical reaction

Physical Chemistry
General104 105 106 110 111 112 116 117 118 122 123 124 Two long straight wires carrying equal currents but in opposite direction are 8 cm apart They produce net magnetic field of 200 T midway between them The magnitude of current in each wire is OPTIONS MARK FOR REVIEW CLEAR SELECTION

Physical Chemistry
Gaseous and liquid states0 In a closed flask of 5 litres 1 0 g of H is heated from 300 to 600 K Which statement is not correct a Pressure of the gas increases b The rate of collision increases c The number of moles of gas increases d The energy of gaseous molecules increases 1991

Physical Chemistry
GeneralWhich one of the following describes better the triple point This point has the highest T and the lowest P in the phase diagram Oa The three phases coexist with a zero degree of freedom O b At the super critical fluid state The three phases coexist with one degree of freedom Od