Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Gaseous and liquid statesincreases 1991 At constant temperature in a given mass of an ideal gas a the ratio of pressure and volume always remains constant b volume always remains constant c pressure always remains constant d the product of pressure and volume always remains constant 1991 The root mean square velocity at STP for the 46 II P mass respe is gi a 47 Press a giv every by de

Physical Chemistry
General47 What amount of energy in KJ is released in the combus 47 tion of 11 6 gm of CH10 9 2CH 9 130 9 BCO 2 0 10H 0AH 5756 KJ 1 575 6 2 287 8 3 182 11 6 TCH109 a difer f K 2CH g 130 g BCO2 g 10H 0AH 5756 KJ 1 575 6 2 287 8 3 182 4 57 56 4 57 56 18 The potential energy of an electron in He is 6 8 eV The 48 Hegfruf 6 8 eva fam

Physical Chemistry
Chemical kineticsK K 3B K3 RDS A If the reaction occurred with individual rate constant K K and K3 and the activation energies associated with them are E 120 kJ mol 1 E3 51 kJ mol 1 and E 210 kJ mol respectively Determine the activation energy of the overall reaction B to C follows first order kinetics Assuming Arrhenius factor for each rate constant and overall rate constant is same 127 kJ mol 1 233 kJ mol 1 93 kJ mol 1 C 210 kJ mol 1

Physical Chemistry
EnergeticsCalculate the heat of formation of ethyl acetate from ethyl alcohol and acetic acid Given that heat of combustion of ethyl alcohol is 34 kcal and of acetic acid it is 21 kcal and of ethyl acetate it is 55 4 kcal 80212

Physical Chemistry
Solid state2 Consider the FeO solid Due to strong heating some Fe ions get oxidised to Fe and the stoichiometry gets disturbed 3 3 It is found that out of 8 iron ions there are 7 Fe and 1 Fe ion present The formula of the given FeO solid should be Fe0 940 Feo 890 Fe0 970

Physical Chemistry
ElectrochemistryFor the following galvanic cell E 0 03 V at 25 C Pt s H g 1 atm H aq 1 M Agt aq 1 M Ag s Calculate the value of AG Ag aq Given 1 F O2895 KJ O 28 95 KJ O 28 95 J 2895 J 96500 C mol

Physical Chemistry
General22 Which of the following statements is wron for gases a Confined gas exerts uniform pressure a the walls of its container in all directions b Volume of the gas is equal to volume c container confining the gas c Gases do not have a definite shape and 3 volume d Mass of a gas cannot be determined by weighing a container in which it i enclosed 1000 29

Physical Chemistry
Equilibriuma 2 30 b 1 50 11 70 29 At 90 C pure water has H 106 M If 100 mL of 0 2 M HCl is added to 200 mL of 0 1 M KOH at 90 C then pH of the resulting solution will be a 5 B 6 c 7 Ho d None of these for the following reaction if the hypochlorous acid solution is diluted will no

Physical Chemistry
ElectrochemistryThe standard electrode potential of Zn Ag and Cu are 0 76 0 80 and 0 34 volt respectively then A Ag can oxidise Zn and Cu B Ag can reduce Zn and Cu2 C Zn can reduce Ag and Cu2 D Cu can oxidise Zn and Ag

Physical Chemistry
General114 Which among the following pairs of reactions has the most favourable equilibrium constant P x CH3CH OH NH3 CH3CH O NH4 y CH3OH NH3 CH3O NH Q x CH3CH OH NH3 CH3CH O NH4 y CH3CH OH CH3NH2CH3CH O 1 P x Q y 2 P x Q x 3 P y Q y 4 P y Q x CH NH

Physical Chemistry
General30 A body of mass 1 kg is executing simple harmonic motion Its displacement y cm at t seconds is given by TC Its maximum kinetic energy is y 6sin 100t 4 a 6 J c 24 J b 18 J d 36 J

Physical Chemistry
General42 A particle of mass 1 kg is undergoing S H M for which graph between force and displacement from mean position is shown Its time period in seconds is a 3 C 6 1 5 13 5 F N 13 5 1 5 b 2 3 d 3 x m

Physical Chemistry
Energetics33 i H g Cl g 2HCl g AH x kJ ii NaCl H SO NaHSO HCI AH y kJ 4 4 iii 2H O 2Cl 4HC1 0 AH z kJ From the above equations the value of AH of HCl is a x kJ b y kJ c z kJ d x 2 kJ

Physical Chemistry
Equilibrium1 Consider the following reaction at 200 C 2CO g O g 2C0 g An equilibrium mixture contains 0 200 mol CO 1 30 mol O and 0 300 mol CO in a 500 ml reactor At a certain instant a valve is opened and 0 100 mol of CO is added to the reactor 1 1 Determine the value of Ke at 200 C

Physical Chemistry
Surface chemistryIn physisorption adsorbent does not show specificity for any particular gas because Involved Van der Waals forces are universal a Enthalpy of adsorption is low Gases involved behave like ideal gases It is a reversible process d O b Oc

Physical Chemistry
GeneralThe activation energy for the forward reaction R P 30 kJ is 40 kJ The activation energy for the reverse reaction is X 70 kJ 10 kJ 70 kJ 10 kJ Solution Answer 2 Ea f 40 Ea b AH 30 AH Ea f Ea b

Physical Chemistry
Generalthen column in a U tube h mb of U tube spring of force constant ation will be ang is assumed to be nstant K when time is noted ot 0 y a cos when time is noted form the extreme position of SHM 1 A body oscillates with SHM according to the equation x TC 5 cos 2 t Its instantaneous displacement at t 4 1 sec is S S com the mean position of SHM 2 b d 5 05

Physical Chemistry
GeneralThe pH value of lemon juice is 3 3 whereas pH of sodium hydroxide is 11 5 So Lemon j uice is acid Sodium hydroxide is a alkaline But We can drink lemon juice which is acidic and can t drink sodium hydroxide which is al kali Why Acid is edible while alkali is non e dible

Physical Chemistry
ElectrochemistryFor the reaction 2H O 2H O AH 571 kJ mol 1 Bond energies of H H and O O respectively are 435 and 498 kJ Then average bond energy of O H bond will be Approx 484 3 390 2 271 4 205

Physical Chemistry
Atomic Structure68 The electron identified by quantum numbers n and 1 1 4 1 1 ii n 4 1 0 iii n 3 1 2 iv n 3 1 1 4 can be placed in order of increasing energy from the lowest to highest b ii iv i iii d iii i iv ii a iv ii iii i i iii ii iv Which represent the correct set up of the four quantum

Physical Chemistry
GeneralOne mole of ideal gas is adiabatically and reversibly 1 compressed to th of its original volume Change 4 in temperature of the gas if initial temperature 27 C given y 1 66 1 100 K 3 449 K 2 200 K 4 350 K the follow

Physical Chemistry
General4 C graphite 4H g CH g 63 Calorific Fuel value of ethane in kJ g if for the reaction 63 2C H 70 4CO 6H 0 AH 745 6 kcal 1 12 4 2 52 3 24 8 4 104 pontaneous 2C 1 3 FAI

Physical Chemistry
General19 According to kinetic theory of gases there are 1 intermolecular attractions 2 molecules which have considerable volume 3 no intermolecular forces of attraction 4 the velocity of molecules decreases for each collision

Physical Chemistry
Generald 3 1 3 A 10 kg metal block is attached to a spring of sp of spring constant 1000 Nm A block is displa from equilibrium by 10 cm and released the maxi acceleration a 10 m s of block is b 100 m s 200

Physical Chemistry
Electrochemistry4 Consider the following polypeptide chain GDAEVKLRIDFW pka a COOH 2 8 pka R arg 11 8 Given pka a NH 9 8 pKa R glu 4 2 pka R asp 3 2 pKa R lys 10 2 What would be the isoelectric point of this polypeptide chain 2 6 46 4 2 44 1 7 00 3 7 46 IAS

Physical Chemistry
Generalenergy 26 When a spring is stretched by 10 cm the potential e stored is E When the spring is stretched by 10 cm more the potential energy stored in the spring becomes b 4E a 2E c 6E d 10E

Physical Chemistry
GeneralA 0 5 gm sample of solid Fe O3 OF 55 2 purity is dissolved in acid and reduced by heating the solution with zinc dust The resultant solution is cooled and made up to 100 ml 75 ml of this solution requires 17 ml of 0 0167M solution of an oxidant for titration The number of electrons in nearest integer taken up by one molecule of oxidant in the above titration is
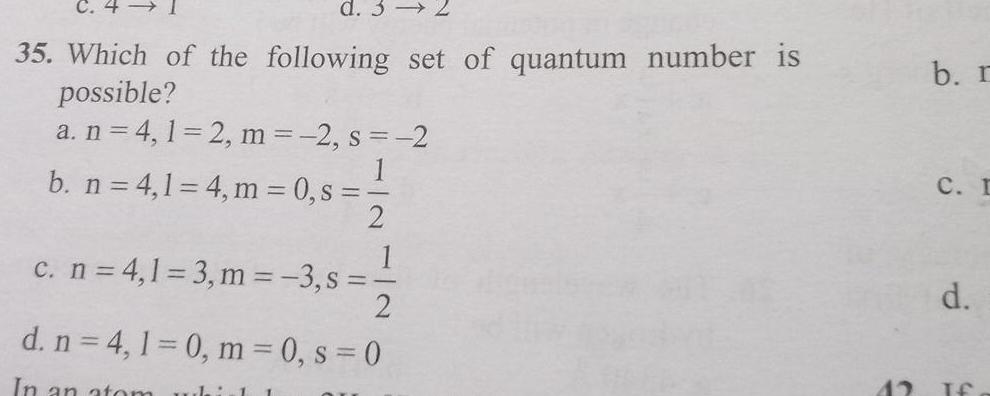
Physical Chemistry
Atomic Structured 3 35 Which of the following set of quantum number is possible a n 4 1 2 m 2 s 2 b n 4 1 4 m 0 s 2 1 c n 4 1 3 m 3 s 2 d n 4 1 0 m 0 s 0 In an atom whirl 1 42 b r C I d If

Physical Chemistry
GeneralA chiral center with R configuration Orotates the propagation plane of light in counter clockwise direction None of the answers provided Is always an L configuration Is always a D configuration the propagation plane of light in clockwise direction

Physical Chemistry
Solid stateIn a unit cell atoms A B C and D are present at the corners face centres body centre and edge centres respectively If atoms along any one of the body diagonal plane of symmetry are removed then find out the formula of compound Question Type Single Correct Type 1 2 3 ABCD2 ABD2 AB2D2

Physical Chemistry
ElectrochemistryThe standard electrode potentials of Zn Ag and Cuare 0 76 0 80 and 0 34 volt respectively then Correct Answer A Ag can oxidies Zn and Cu B Ag can reduce Zn and Cu Your Answer C Zn can reduce Ag and Cu D Cu can oxidise Zn and Ag

Physical Chemistry
Surface chemistry57 The langmuir adsorption isotherm is deduced using the assumption A The adsorption sites are equivalent in their ability to adsorb the particles B The heat of adsorption varies with coverage C The adsorption molecules interact with each other D The adsorption takes place in multiplayers

Physical Chemistry
Energetics5 The value of AH in kJ for the reaction will be CS 1 4NOCI g CCI 1 2SO g 2N g if AH CS x AH CO z 1 x 4y z 2r AH NOCI Y AH SO r 2 r z 4y X 3 2r z 4y x 5 Wyd

Physical Chemistry
Gaseous and liquid states4 A closed flask contains water in all its three states solid liquid and vapour at 0 C In this situation the average kinetic energy of water molecules will be a the greatest in all the three states b the greatest in vapour state c the greatest in the liquid state d the greatest in the solid state 1992

Physical Chemistry
Gaseous and liquid states164 At low pressures For 1 mole the Vander Waal s equation is written as a P The compressibility factor is then equal to 1 RTV A C 1 V RT a RTV a RTV B D 1 RTV a

Physical Chemistry
GeneralWhich characteristic s of rems compound s below is are accurate The chiral centers in miso compounds possess the exact same set of substituents Meso compounds possess chiral centers but the rotations completely cancel each othe out All of the listed characteristics are accurate Meso compounds display zero net rotation of miso compounds has an internal plane of symmetry

Physical Chemistry
General5 A simple pendulum of frequency n falls freely unc gravity from certain height from the ground level frequency of oscillation a Remain unchanged b n 2 c Becomes zero d Becomes infinit

Physical Chemistry
General29 The displacement of a particle executing SHM is given where y is in meter at time t in c 0 25 T by y 10sin 6t 3 d seconds The initial displacement and velocity of the particle are respectively 5 3 m and 30m sec a b 15m and 5 3m sec c 15 3 m and 30m sec C sec 10 3 2 m and 30m sec

Physical Chemistry
Gaseous and liquid statesZero darkened Negative Marks 1 In all 1 Combustion of 50 mL methane is carried with 150 ml of air containing 21 of oxygen What will be the total volume of gaseous mixture after reaction CH 20 CO g 2H O g 2 8 2 110 mL 4 144 5 mL 58 1 200 ml 3 113 mL The mass of glucose that would be dissolved of water in order to produce the same pressure as is produced by the same quantity

Physical Chemistry
GeneralCaustic soda NaOH can be prepared commercially by the reaction of Na CO3 with slaked lime Ca OH 2 How many gram of NaOH can be obtained by treating 1 06 kg of Na CO3 with 1 48 kg of slaked lime Na CO3 Ca OH 2NaOH CaCO3

Physical Chemistry
GeneralThe term Racemic refers to Any mixture of enantiomers that displays no net optical activity A 50 50 mixture of enantiomers that displays no net optical activity Any mixture of diastereomers that displays no net optical activity A 50 50 mixture of diastereomers that displays no net optical activity

Physical Chemistry
EnergeticsWhich of the following relation is correct Question Type Single Correct Type 0 1 AH H 0 l AHC H g 2 AH CO g AHCO C graphite 3 4 AH CO g AH C AH CO g All of the above Your Answer 3 Status Incorrect graphite

Physical Chemistry
Energetics165 A box of 1L capacity is divided into two equal compartments by a thin partition which are filled with 2g H and 16gm CH4 respectively The pressure in each compartment is recorded as P atm The total pressure when partition is removed will be A P C P 2 B 2P D P 4

Physical Chemistry
SolutionsA 0 002 molar solution of a complex Pt NH3 4C14 in water had a freezing point depression of 0 0108 C Given K for H 0 1 86K molality Considering 100 ionization of the complex how many ions are produced after ionization

Physical Chemistry
Atomic StructureWhich one of the following is not the characteristic of Planck s quantum theory of radiation 1 The energy is not absorbed or emitted in whole number multiple of quantum 2 Radiation is associated with energy 3 Radiation energy is not emitted or absorbed continously but in the form of small packets called quan 4 This magnitude of energy associated with a quantum is proportional to the frequency Calculate the energy of a photon of sodium light of wave length 5 826 10 16 m in Jules

Physical Chemistry
GeneralIf enantiomer A has 3 chiral centers a R configuration b R configuration and c S configuration which have specific rotations of 33 0 20 5 and 32 0 respectively what will the observed rotation for enantiomer A be 44 5 degrees 85 5 degrees O 85 5 degrees 445 degrees

Physical Chemistry
Gaseous and liquid statesme spontaneou 1 The solubility of NaCl in water at 25 C is about 6 moles per litre Suppose you add 1 mole of NaCl to a litre o water For the reaction NaCl H O Salt solution a AG 0 AS 0 c AG 0 AS 0 b AG 0 AS 0 d AG 0 AS 0

Physical Chemistry
ElectrochemistryGiven E Zn 2 Zn K Cu NH3 4 2 4 10 1 0 34 V Cu 2 Cu 0 76 V Zn electrode 1 L 2 M ZnSO4 Answer the following Cu electrode 1 L 0 2 M CUSO4 61 The emf of cell at 200 K is Given 2 303 R 2 10 4 and assume that E values are independent on temperature a 1 7 V c 1 09 V 1 08 V d 1 10 V 62 When 1 mole NH3 added to cathode compartment then emf of cell is at 298K a 0 81 V c 1 1 V b 1 91 V d 0 72 V 63 At what conc of Cut2 emf of the cell will be zero a 298K and conc of Zn 2 is remaining same 110 x 10 37 h 1 19 x 10 20

Physical Chemistry
GeneralThe correct statements about production of Br are A The pH of the sea water is raised to about 8 5 B The pH of the sea water is lowered to about 3 5 C An excess of chlorine is bubbled through the sea water D Br and BrO3 ions can be converted to Br by oxidation with Cl H SO4 O A and D O A C and D OB C and D A and C

Physical Chemistry
Gaseous and liquid statesThe compression factor Z of certain a gas is equal to 0 83 when the temperature is 315 K and the pressure is 23 atm How much volume 5 7 mmol of the will occupy under these conditions