Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
SolutionsIdentify the incorrect statement s in volume of solution On adding HCI to NaOH aqueous solution the boiling point of the solution decreases initially and then gradually increases On adding NaOH to Ca OH 2 aqueous solution the freezing point increases initially On adding HCI to NaOH aqueous solution the relative lowering in vapour pressure increases initially then decreases On adding water to a solution containing 0 1 M NaOH and 0 1 M HCI boiling point of

Physical Chemistry
General19 4 I A ns np5 B n 1 d 0 ns C n 1 d5 ns D n 1 d 0 ns np6 1 A q B s C p D r 2 A R C 4 II P 9 R r for s n 4 2 A s B q C p D r AY

Physical Chemistry
Equilibriuma 10 b 10 5 35 What is the percent dissociation a of a 0 01 M HA solution K 104 c 10 d 10 0 a d 17 a 9 5 b 1 c 10 5 36 Given the two concentration of HCN K 10 are 0 1 M and 0 001 M respectiv

Physical Chemistry
Atomic StructureSir why not answer should be option 4 correct 45 180 The Bohr model for the spectra of a H atom Will be applicable to hydrogen in the molecular form Will not be applicable as it is for a He atom Is valid at room temperature only Predicts continuous as well as discrete

Physical Chemistry
Atomic Structureemitted out to the number of quanta absorbed A photon of 300 nm is absorbed by a gas and then re emits two photons One re remitted photon has wa length 500 nm Calculate energy of other photon re emitted out

Physical Chemistry
Solid statean element X has two crystalline forms P CSCI type and Q ZnS type zinc blende in which both the positions of cations and anions are occupied by the atoms o element X dentify the correct statement s Ratio of mass of unit cell of P type and of Q type crystalline form is 4 The ratio of packing fraction of P type and Q type crystalline form is 2 approximately The ratio of packing fraction of P type and Q type crystalline form is 4 approximately The ratio of density of P type and Q type crystalline form is 2 approximately

Physical Chemistry
Equilibrium3 Acetic acid K 1 8 x 10 4 Benzoic acid K 6 5 10 The pH of 0 1 M monobasic acid is 4 50 The acidity constant of the monobasic acid is 1 1 0 x 10 7 2 1 0 x 10 3 1 0 104 4 1 0 10

Physical Chemistry
GeneralWhich one is a wrong statement 2018 a Total orbital angular momentum of electron in s orbital is equal to zero b An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers c The value of m for d is zero d The electronic configuration of N atom is 1s 2s 2s 2p 2p 2p 1 1 114

Physical Chemistry
ElectrochemistryA current of 9 65 A is passed through the aqueous solution of NaCl using suitable electrodes fo 1000 s The amount of NaOH formed during electrolysis is 1 2 0 g 2 4 0 g 3 6 0 g 4 8 0 g 21

Physical Chemistry
Equilibriumc 5 4 x 10 5 4 x 10 23 pH of 10 6 M HCl aq is a just less than 6 c just greater than 6 Med d 8 1 x 10 3 8 1 x 107 3 b exactly equal to 6 d just less than 7 24 10 5 M NaOH solution at 25 C is diluted 1000 times The pH of the resultant solutio

Physical Chemistry
Energetics270 Given C s O2 g CO2 AH 94 2 K Cal H g 1 2O2 g H OAH 68 3 K Cal CH4 8 202 g CO2 g 2H O AH 210 8 K Cal what will be heat of formation of CH in K Cal 1 45 9 2 20 0 3 47 8 4

Physical Chemistry
General3 Ag s 4 HBr aq 644 in the given rex the HCHO 1 2 Ag NH aq 3OH aq 2Ag s reducing HCOO aq 4NH aq 2H O 1 1 HCHO 1 3 HCOO aq 2 Ag NH3 aq 4 NH aq

Physical Chemistry
GeneralIf S S S and S are the solubilities of AgCl in water 0 01 M CaCl 0 01 M NaCl and 0 5 M AgNO solutions respectively then which of the following is true 2 S S S S 4 S S S S 1 S S S S 3 S S S S

Physical Chemistry
General26 What will be the output of the following C code include int main int i 23 char c 23 if i c printf Yes n else printf No n Yes Depends on the compiler Depends on the standard

Physical Chemistry
ElectrochemistryMolar conductances of BaCl H SO and HCl at infinite dilutions are x x and x respectively Equivalent conductance of BaSO at infinite dilution will be 1 1 X1 X2 X3 2 X X X 2 3 2 x x 2x 4 X x 2x 2

Physical Chemistry
Solid state9 In a NaCl structure if positions of Na atoms and Cl atoms are interchanged then in the new unit cell 1 Na atom is present at body centre 2 Cl atom is present at face centre 3 Na atom is present in tetrahedral voids 4 Clatom is present in octahedral voids

Physical Chemistry
ElectrochemistryCalculate E for the following cell cell BEGINNER S BOX 2 Zn s Zn Ag Ag s E 1 0 04 V 2 1 56 V 0 76V E 0 80V Ag Ag 3 1 56 V 4 0 84 V

Physical Chemistry
Solid stateThe correct statement regarding defects in Re AIPMT 2015 crystalline solids is 1 Frenkel defect is a dislocation defect 2 Frenkel defect is found in halides of alkaline metals 3 Schottky defects have no effect on the density of crystalline solids 4 Frenkel defects decrease the density of J US

Physical Chemistry
GeneralAccording to third law of thermodynamics the entropy at 0 K is zero for a Elements in their stable form b Perfectly crystalline solids c Substance as 1 atm and 25 C d N 0

Physical Chemistry
EnergeticsWhen the formation of one mole HBr g takes place reversibely from gaseous H and liq Br2 at 300K and 1 atm increase in entropy of the surrounding is 170 J K What is change in entropy J K of the system if reaction 2HBr g H g Br2 1 is carried out reversibly at 300K in rigid container Given R 8 3 J mol K

Physical Chemistry
GeneralThe heat of neutralisation of four acids A B C and D when neutralised against a common base are 13 7 9 4 11 2 and 12 4 kCal respectively The weakest among these acids is a A c C b B d D

Physical Chemistry
Atomic Structure8 The second order Bragg diffraction of X rays with 1 00 A from a set of parallel planes in a metal occurs at an angle 60 The distance between the scattering planes in the crystal is 1 2 00 A 2 1 00 A 3 0 575 A 4 1 15 A

Physical Chemistry
Chemical kineticsCalculate the rate of the reaction CH3CO 0 H O 2CH3COH 1 if its rate constant is equal to 1 0 0454 min the concentration of acetic anhydride is equal to 0 45 mol l The reaction is first order with respect to acetic anhydride zero order for water

Physical Chemistry
Solid stateA crystal may have one or more planes o symmetry as well as one or more than one axis a symmetry but it has 1 Two centres of symmetry 2 Only one centre of symmetry 3 No centre of symmetry 4 Three centres of symmetry

Physical Chemistry
EquilibriumWhich of the following is the weakest acid 1 Phenol K 1 3x10 0 3 Acetic acid K 1 8 x 10 2 Hydrocyanic acid K 4 9x10 0 4 Benzoic acid K 6 5 x 10

Physical Chemistry
Equilibriumx y S xy 3 K x y S 4 K x y S The molar solubility of silver sulphate is 1 5 x 102 mol L The solubility product of the salt will b 1 2 25 x 104 2 1 35 x 10 5 3 1 7 x 10 4 3 0 x 10

Physical Chemistry
EnergeticsThe equilibrium constants of a reaction at 298 K and 308 K are 1 0 10 and 2 x 10 respectively the reaction is a Exothermic b Endothermic c May be endothermic or exothermic d Cannot be predicted

Physical Chemistry
Solid stateA given metal crystallizes out with a cubic structure having edge length of 361 pm If there are four metal atoms in one unit cell what is the radius of one atom AIPMT 2015 1 108 pm 3 127 pm 2 40 pm 4 80 pm

Physical Chemistry
General2 15 9 g 4 63 5 g A quantity of electric charge that brings about the deposition of 4 5 g Al from Al at the cathode will at produce the following volume STP of H g from H at the cathode 3 11 2 L 1 44 8 L 2 22 4 L 4 5 6 L

Physical Chemistry
GeneralWhat happens when sodium is added to water A gas is evolved II The temperature of the water increases III A clear colourless solution is formed A I and II only B I and III only C II and III only D I II and III 1

Physical Chemistry
Nuclear chemistryCalculate the wavelength of light required to break the bond between two bromine atom in bromine molecule The Br Br bond energy is 93 kJ mol Take N 6x 1023 h 6 6 10 34 J s A 1 27 x 106m B 1 26 10 m C 1 8 10 20 m D 1 9 10 13 m The radius ratio of two orbits of Li2 is 1 16 What is the ratio of frequency of revolution of electron in these orbits

Physical Chemistry
GeneralIn which of the following chemical equations the abbreviations represent the correct states of the reactants and products involved at reaction temperature a 2H2 g 02 g 2H2O 1 b 2H2 g 02 1 2H2O 1 c 2H2 1 02 1 2H2O g d 2H2 g 02 g 2H2O g 8 41 PM

Physical Chemistry
ElectrochemistryThe ionization constant of a weak electrolyte is 25 x10 while the equivalent conductance of its 0 01 M solution is 19 6 S cm eq The equivalent conductance of the electrolyte at infinite dilution in S cm eq will be 1 39 2 2 78 4 3 392 4 196 0 21

Physical Chemistry
Solutions2 Which of the following solutions cannot act as a buffer system 1 KH PO H PO 2 NaCIO HCIO A buff 3 C H N CH NH 4 Na CO NaHCO

Physical Chemistry
Chemical kinetics3 The half life of a substance is the length of time for an unstable element to spontaneously decay to one half of its original amount Plutonium 239 has a half life of 24 years The formula for half life is as follows M 100 0 5 Determine how long it would take for the sample to decay to only 5 0 g 31

Physical Chemistry
GeneralThe wavelength in A of an emission line obtained for Li during a electronic transition from n 2 to n 1 is R Rydberg constant a 3R 4 b 27R 4 c 4 3R d 4 27R

Physical Chemistry
GeneralGurukripa an example of redox Rexn following rexn is not CAREER INSTITUTE 29 34 1 H PO aq 4AgNO3 aq 2H O 1 H PO4 aq 4Ag s 4HNO3 aq 2 N H 1 2H O 1 N g 4H O 1 3 2 K s F g 2KF s 4 BaCl aq H SQ g 35

Physical Chemistry
General6 1 16 If 0 224 L of H gas is formed at the cathode the volume of O gas formed at the anode under identical conditions is 1 0 224 L 2 0 448 L COMMERCIAL VOLTAIC CELLS 3 0 112 L 4 1 12 L

Physical Chemistry
ElectrochemistryMethane gas and steam in equimolar amounts are taken in a flask and sealed where the following equilibrium was established CH g H O v CO g 3H g 2H g CO g CH OH g K atm At equilibrium if partial pressure of CO g as found to be 0 5 atm and total pressure inside the flask was 5 atm then K of the first reaction is approximately A 3 5 atm B 7 5 atm C 10 5 atm D 12 5 atm

Physical Chemistry
General16 The solubility of AgCN in a buffer solution of pH 3 is x The value of x is Assume No cyano complex is formed K AgCN 2 2 10 16 and K HCN 6 2 10 10 sp 2 1 9 x 10 5 4 1 6 x 10 6 1 0 625 x 10 6 3 2 2 x 10 16

Physical Chemistry
Generalc CH3COOH 4 7 33 Given Enthalpy of ionization of two acids AH HCN 45 2 kJ mol AH CH3COOH 2 1 kJ mol Which relationship for the two acids is true a pK HCN pK CH3COOH pK HCN PK CH3COOH 1 What is the hydronium ion concentrat d CH3CH COOH 4 88 b pK HCN PK CH3COOH CE 45 2 d pK HCN 2 1 PK CH3COOH

Physical Chemistry
Gaseous and liquid statesN2 3H 2NH3 1 mole of N and 4 moles of H are taken in 15 L flask at 27 C After complete conversion of N into NH3 5 litres of water is added What is the pressure set up in the flask A B G 4 926 atm 3 284 atm 1 643 atm

Physical Chemistry
GeneralThe photograph shows silica packets which are used to absorb moisture in the packaging of certain products How did the silica minerals in these packets form y DO NA ROSA AWE GEL OA By the chemical precipitation of sedimentary rock O B By the weathering of sediment OD By the crystallization of magma C By the melting of metamorphic rock

Physical Chemistry
GeneralTwo acid HX and HY have strength ratio 5 2 their heat of neutralization are in the ratio of 5 4 If 40 gm of NaOH is added to a mixture of acids containing total 1 equivalent of HX and HY The total heat evolved is Q What is the ratio of Q and Q2 where Q is heat of neutralization of HY 1

Physical Chemistry
Gaseous and liquid statesDetermine the equilibrium constant of the following reaction CO g 2H g AH 128 kJ mol The equilibrium concentrations of CO H and CH3OH are 0 02 M 0 06 M and 0 01 M respectively CH OH CH3OH g Define the shift of the equilibrium for the following reaction in case of a Increase of temperature Explain your answer b Decrease of pressure Explain your answer

Physical Chemistry
Atomic StructureThe magnitude half of the orbital angular momentum vector of an electron in an orbital is expressed as Vo Into how many components will the vector split if a magnetic field is applied on it Rate this question Your Answer 2
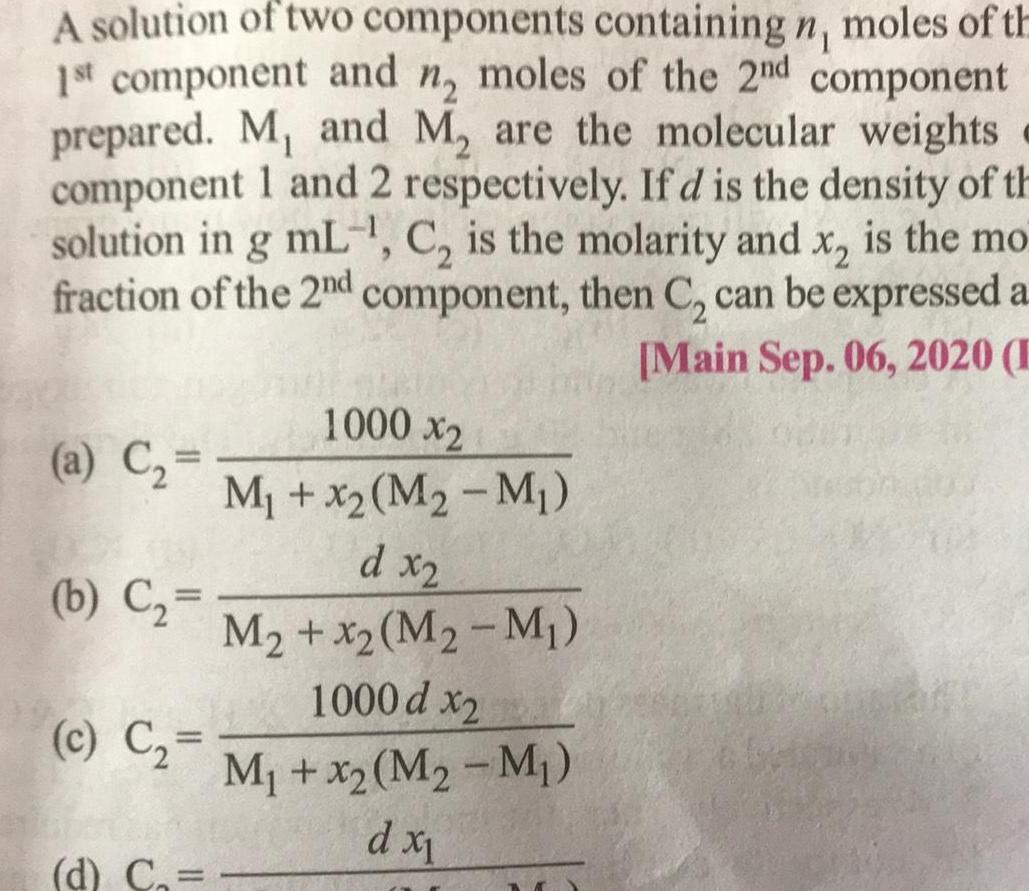
Physical Chemistry
SolutionsA solution of two components containing n moles of th 1st component and n moles of the 2nd component prepared M and M are the molecular weights component 1 and 2 respectively If d is the density of th solution in g mL C is the molarity and x is the mo fraction of the 2nd component then C can be expressed a Main Sep 06 2020 E a C b C c C 1000 x2 M x M M d x2 M x2 M M 1000 d x2 M x2 M2 M d x d C

Physical Chemistry
Solutionsb A piece of wood that measures 3 2 cm by 5 7 cm by 7 3 cm has a mass of 100 g What is the density of the wood Would it float on water 7 Known Values 1 Formula s and Equation s 5 Final Statement 1

Physical Chemistry
Gaseous and liquid states108 The volume vs temperature graph of 1 mole of an ideal gas is given below X 50 Volume L 40 30 20 100 Z 200 300 400 Y 500 Temperature K The pressure of the gas in atm at X Y and Z respectively are 0 328 0 820 0 820 b 3 28 8 20 3 28 c 0 238 0 280 0 280

Physical Chemistry
Chemical kinetics33 The result of three experiments carried out for etermination of differential rate of reaction Cl2 g 2NO g i Mention differential rate law for reaction ii Mention order of reaction iii Find value of specific rate constant Experiment number 2NOCI are as follows then 1 2 3 Initial concentration mole litre Cl 0 06 0 06 0 02 NO 0 03 0 08 0 08 S L Section D 8 4 marks Initial rate of reaction d Cl dt mole litre sec 0 0054 0 0384 0 0128