Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Equilibriumlle between 6 and 7 d remain unchanged 25 4 0 g of NaOH and 4 9 g of H SO4 are dissolved in water and volume is made upto The pH of this solution is a 7 0 c 2 0 d 12 0 b 1 0

Physical Chemistry
Chemical BondingThe correct order of E values with negative sign M IM for the four successive elements Cr Mn Fe and Co is a Cr Mn Fe Co b Mn Cr Fe Co c Cr Fe Mn Co d Fe Mn Cr Co the decomposition of Al2O

Physical Chemistry
General215 H has two natural isotopes of H and H and 0 has C v two isotopes 0 6 and O18 Which of the following mol mass of H O will not be possible a 19 24 b 20 d 22 o wave equation of an elect

Physical Chemistry
Atomic StructureThe radial part of schrodinger wave equation for hydrogen atom is 1 0 2 y r 16 4a 2 1 0 o 80 12 e Where a constant o 2r na n principle quantum number Select the correct statements A Distance of nearest radial node from the nucleus is 2a B Distance of farthest radial node from the nucleus is 12a C Number of maxima in the curve y r vs r are 4 n r is for 4n orbital

Physical Chemistry
Gaseous and liquid statesCl O gas decomposes as Cl 07 g Cl g O g A partially decomposed gaseous mixture is allowed to effuse through a pin hole and the gas com out initially was analysed The mol fraction of the O was found to be 0 6 determine the degree dissociation a 40 b 30 c 20 d 10

Physical Chemistry
GeneralCAREER INSTITUTE 59 The V C distance in V CO and V CO 6 are respectively 59 V CO V CO V pound anmics in pm 1 200 200 2 193 200 3 200 193 4 193 193 conci C W Isomerism IUP 1 200 200 2 193 200 3 200 193 4 193 193 V C B

Physical Chemistry
General30 What change will occur for the following reaction if the hypochlorous acid solution is diluted from 0 1 to 0 01 M HOCl aq H O 0 OCl aq H O aq a a decrease in the fraction of acid ionized b an increase in the fraction of acid ionized c no change in the fraction of acid ionized d we can not predict Given 1

Physical Chemistry
GeneralA hydrocarbon contain 80 C The vapour density of compound is 30 Empirical for compound is 1 CH 2 C H 4 CH Two elements X Atomic weight 75 and Y Atomic weight 16 combine to give a compound having 75 89 of X The empirical formula of compound is 1 XY 2 X Y 3 X Y XY

Physical Chemistry
Electrochemistry7 Standard free energies of formation in kJ mol at 298 K are 237 2 394 4 and 8 2 for H O CO2 g and pentanerespectively The value of E cell for the pentane oxygen fuel cell is a 1 0968 V c 1 968 V b 0 0968 V d 2 0968 V 2008

Physical Chemistry
GeneralThe curve in the figure shows the variation of pH during the course of titration of a weak acid HA with a strong base NaOH At which point in the titration curve is the concentration of the acid may be equal to that of its conjugate base pH A B a Point D c Point C D C E ml of NaOH added b Point E d Point B

Physical Chemistry
Solutions68 KBr is 80 dissociated in aqueous solution 68 0 5milar ca faca KBr 80 fa refer K 1 86 K kg mol of 0 5m concentration Given K for water 1 86 K kg mol The solution freezes at 1 271 326 K 3 270 5 K 2 272 K 4 268 5 K fer 1 271 326 K 3 270 5 K 2 272 K 4 268 5 KM

Physical Chemistry
Chemical kineticsIn a reaction A B Product rate is doubled when the concentration of B is doubled and rate increases by a factor of 8 when the concentrations of both the reactants A and B are doubled rate law for the reaction can be written as AIRMT Prelims 20121

Physical Chemistry
Chemical BondingMatch the Xenon compounds in Column I with its structure in Column II and assign the correct code NEET 2019 Column l a XeF b XeF c XeOF d XeO3 Code a Column II i Pyramidal ii Square planar iii Distorted octahedral iv Square pyramidal b c b d iii iv iii iv i iii i iv iv i ii 1 i ii 2 ii 3 ii 4 iii

Physical Chemistry
General7 Different hydrogen in the compound are 77 faf gten vergel at diferente a represented by alphabets CH CH CH CH CH CH CH D E F A B C arrange them in decreasing order of reactivity towards free radical substitution 1 C A E D F B 2 F B A C D E 3 B C A F D E 4 A B C D E F CH CH CH CH CH CH CH B C D E F segur pasi fisuriteam A 1 C A E D F B 2 F B A C D E 3 B C A F D E 4 A B C D E F

Physical Chemistry
Solid stateConsider the three axis of symmetry mentioned below in a cubic close packing i C 2 ii C 3 iii C 5 Which of the given axes of symmetry lie on the body diagonal plane i and ii only ii and iii only i and iii only i ii and iii

Physical Chemistry
General4 2 1 80 g of a certain metal burnt in oxygen gave 3 0 g of its oxide 1 50 g of the same in steam gave 2 50 g of its oxide Show that these results illustrate the law of constant proportion 1 In the first sample of the oxide

Physical Chemistry
Equilibrium25 ml of a 0 1 M solution of a stable cation of transition metal z reacts exactly with 25 ml of 0 04 M acidified KMnO4 solution Which of the following is most likely to represent the change in oxidation state of z correctly B C Z Z2 Z2 Z3 Z3 24 22 24 J X

Physical Chemistry
Atomic StructureUsing arbitrary energy units we can calculate that 864 arbitrary units a u are required to transfer an electron in hydrogen atom from the most stable Bohr s orbit to the largest distance from the nucleus n E 0 n 1 E 864 Arbitrary units The energy required to transfer the electron from third Bohr s orbit to the orbit n will be 1 96 Arbitrary units 2 192 Arbitrary units 3 288 Arbitrary units 4 384 Arbitrary units
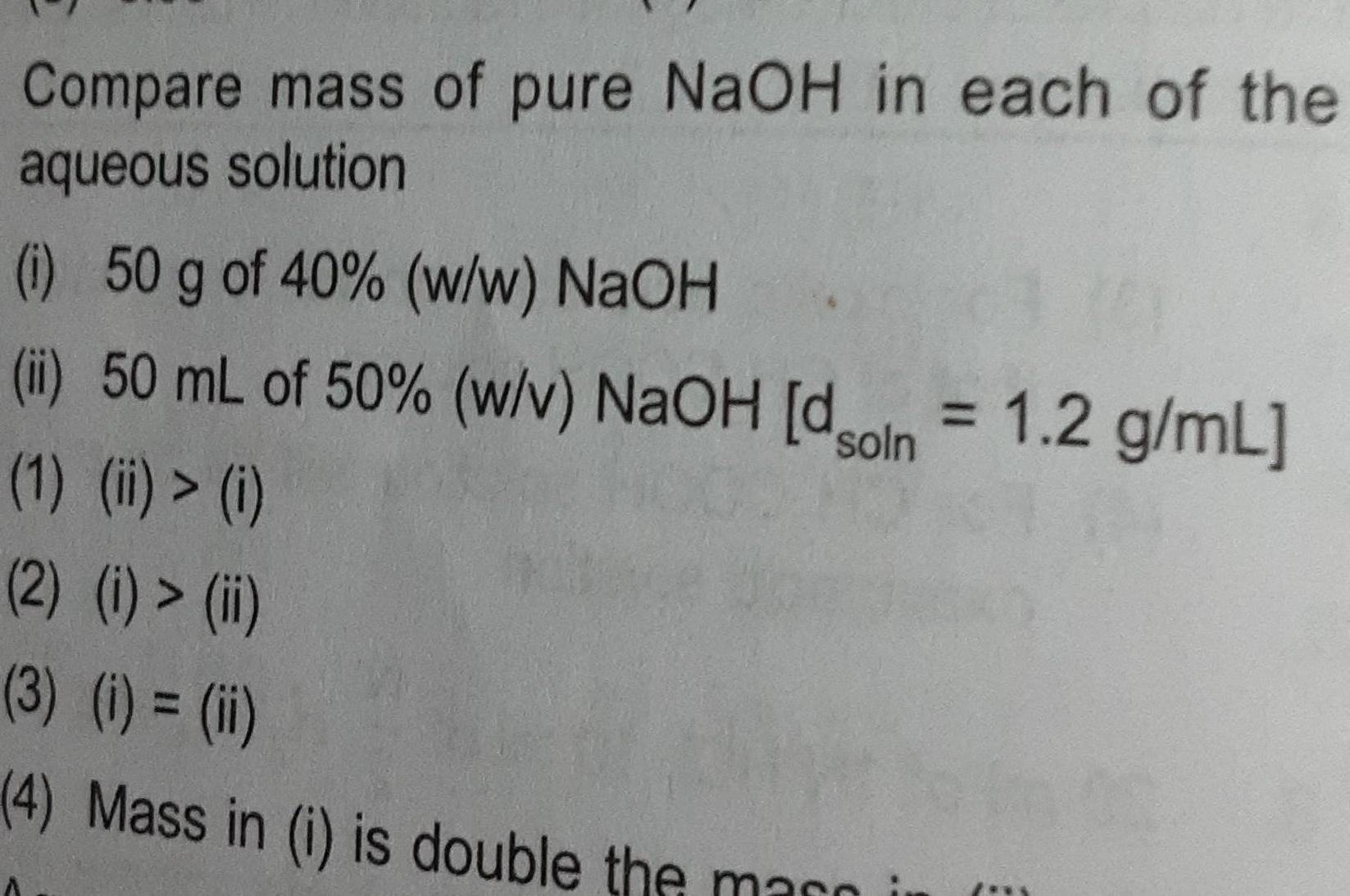
Physical Chemistry
Chemical kineticsCompare mass of pure NaOH in each of the aqueous solution i 50 g of 40 w w NaOH ii 50 mL of 50 w v NaOH dson 1 2 g mL 1 ii i 2 i ii 3 i ii 4 Mass in i is double the marni

Physical Chemistry
Gaseous and liquid states2 Copper forms two oxides For the same amount of copper twice as much oxygen was used to form the first oxide than to form the second one What is the ratio of the valencies of copper in the first and second Oxides Hint Assume that the oxides are Cu O and Cu O and apply Rule 6 2 1

Physical Chemistry
Surface chemistryWhich of the following is correct for Langmuir s adsorption isotherm W extent of adsorption i e x m ap 1 bP A X m W Plot of P W 12 All of these a 1 versus P has as intercept a

Physical Chemistry
ElectrochemistryA current of 0 1A was passed for 2hr through a solution of cuprocyanide and 0 3745 g of copper was deposited on the cathode Calculate the current efficiency for the copper deposition Cu 63 5 O O O 79 39 5 63 25 62 5

Physical Chemistry
Surface chemistryWhich of the following phenomenon occurs when a chalk stick is dipped in ink A Adsorption of coloured substance B Adsorption of solvent C Absorption and adsorption both of solvent D Absorption of solvent

Physical Chemistry
ElectrochemistryGiven that E Ag aq Ag s 0 80 V E Ni aq Ni s 0 25 V the value of E corresponding to the reaction cell 2Ag aq 0 01 M Ni s 2Ag s Ni aq 0 0001M is 2 A 0 991V B 1 05V C 0 55 V D 1 70 V

Physical Chemistry
General4 B Be N 0 C A B Cation Anion XeF SbF Incorrect statement for above changes is 1 XeF act as F donor 2 Hybridisation state of central atom in cationic part is sp d 3 Anionic part is isostructural with PCI 4 Both cation Anion have identical shape 4 B Be N 8 C A Cation 47 XeF SbF B Anion Buda ufada fast 1 XeF Fal si are si sa 2 3 ten sp d C 4 A fa t

Physical Chemistry
Chemical kinetics47 Minimum amount of Ag CO s required to produce 47 Ag CO s Cuide de fac S T P CO sufficient oxygen for the complete combustion of C H which produces 11 2 ltr of CO at S T P after combustion is Ag 108 Ag CO3 s 2Ag s CO g 1 2O g C H 5 20 2CO H O 1 276 g 11 2 2 345 g 227 Ag cu 3 690 g 4 1380 g 100 11 2 Ag 108 Ag CO s 2Ag s CO g 1 2O g C H 5 20 2CO H O 1 276 g 2 345 g 3 690 g 4 1380 g

Physical Chemistry
General31 The nucleus of an atom of X is supposed to be a sphere with a radius of 5x 10 cm Find the density of the matter in the atomic nucleus if the atomic weight of X is 19 Hint Density mass of 1 mole i e at wt vol of 1 mole 6 02 x 10 g mL

Physical Chemistry
Electrochemistry3 Atoms consist of electrons protons and neutrons If the mass attributed to neutron was halved and that attributed to the electrons was doubled the atomic mass of 6C 2 would be approximately a same c halved b doubled d reduced by 25 hos the electronic configuration 18 Az

Physical Chemistry
EquilibriumThe reaction 2A g B g 3C g D g is begun with the concentrations of A and B both at an initial value of 1 00 M When equilibrium is reached the concentration of D is measured and found to be 0 25 M The value for the equilibrium constant for this reaction is given by the expression AIPMT Mains 2010 1 0 75 0 25 1 00 1 00 2 0 75 0 25 0 50 2 0 75 3 0 75 0 25 0 50 0 25 4 0 75 0 25 0 75 0 25

Physical Chemistry
Atomic StructureA glow worm of mass 5 0 g emits red light 650 nm with a power of 0 1 W entirely in the backward direction What velocity would it accelerate to after 10 years if released in free space and assumed to be alive 1 9 14 ms 1 2 28 08 ms 1 3 11 05 ms 1 4 21 04 ms 1

Physical Chemistry
GeneralAr Haut is AA fatfed fand A It is possible to differentiate the two isomers of the 1 3 A complex Co NO 3 NH3 3 by comparing their dipole moment values R The complex Co NO 3 NH3 3 shows facial and meridional isomerism Only cis PHNH Q R High Co NO NH3 3 facial en meridional R

Physical Chemistry
EnergeticsMatch List I with List II and select the correct answer using the codes given below the lists List I G P G A B C D T P H T A F a 5 b 5 c 3 P H D B C 1 2 4 3 2 4 5 2 1 List II 1 MJT 2 T 3 S 4 P 5 V 2010

Physical Chemistry
SolutionsA 25 mL 0 05 M HCl solution was mixed with 75 00 mL 0 01 M KOH solution 20 mL of the resulting solution was titrated for neutralization using a standard Ba OH solution and its 25 mL was required The molarity of Ba OH solution is a 0 004 M b 0 002 M c 0 001 M d 0 02 M

Physical Chemistry
Atomic StructureSelect INCORRECT statement about the following molecular orbital O It is a bonding molecular orbital It is formed by overlapping of lobes of p and d2 which are in same phase Two atoms approach along z axis in order to form bond O Centre of symmetry is present in it

Physical Chemistry
Generalgura 10 r 11 1g H gas STP is expanded so that Hence work done is 1 22 4 L atm 2 5 6 L atm 3 11 2 L atm 4 44 8 L atm Given msat SNULL 3 X2 LIO 300R 303 101 6 X101 606 x 1 yoxxy 83 22 kcal A B es una aldi faal PV 1x Ray 22 9 1 22 4 L atm 2 5 6 L atm 3 11 2 L atm 4 44 8 L atm 55 f r 49 2214 PAV 2N V 1 2 1 atm astea N g 3H g 2NH g AH 22 kcal

Physical Chemistry
General75 00 8 Taxable Amount NCode 6307 Total Gross Discount SplDiscount SchemeDisc B4 Taxaros Total CAST op Net Amt 4 17 4 17 175 00 00 66 67 8 34 SLIE CGST SGST TotalTax Amount 4 17 4 17 1 75 00 00 00 00 8 34 8 34

Physical Chemistry
Chemical BondingThe cell potential for the voltaic cell depicted below is 0 109V under standard condition 2 2 Pb s Pbq Niaq Ni s aq Which change will decrease the voltage A The 1 M Ni2 solution is diluted with water B A larger Ni electrode is used C Cl ions is added to precipitate PbCl s D2M Pb2 solution is added to lead half cell tom ia

Physical Chemistry
GeneralWhen a quantity of electricity equal to that required to liberate 2 24 L of hydrogen at STP from 0 1 M aqueous H SO4 is passed then the mass of copper that will be deposited at cathode in electrolysis of 0 2 M solution of copper sulphate will be At mass of Cu 63 5 Question Type Single Correct Type 1 2 3 1 59 g 3 18 g 6 35 g

Physical Chemistry
Atomic StructureThe average atomic weight of copper which has two naturally occurring isotopes is 63 5 One of the isotopes has an atomic weight of 62 0 amu a constitutes 69 1 of the copper isotopes The other isotope has an abundance of 30 9 The atomic weight amu of the second isotope is 5 X amu
Physical Chemistry
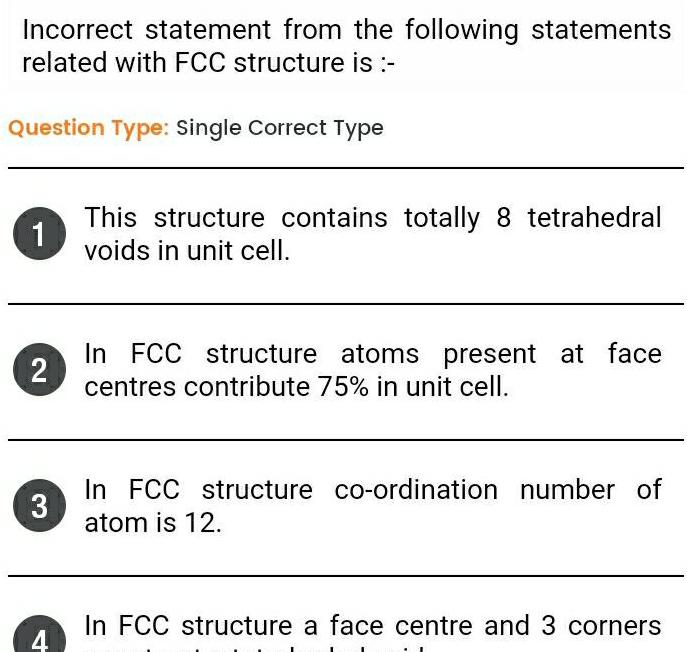
Solid stateIncorrect statement from the following statements related with FCC structure is Question Type Single Correct Type 1 This structure contains totally 8 tetrahedral voids in unit cell 2 In FCC structure atoms present at face centres contribute 75 in unit cell 3 In FCC structure co ordination number of atom is 12 In FCC structure a face centre and 3 corners 4

Physical Chemistry
Equilibrium2 10 c 0 02 d 0 1 149 What mass of AgI will dissolve in 1 0 L of 1 0 M NH 3 Neglect change in conc of NH 3 Given Ksp Ag 1 5x 10 6 K Ag NH3 21 1 6 x 107 At mass Ag 108 1 127 b 0 0056 g c 0 035 g d 0 011 g a 4 9 x 10 5 g 150 Consider the following statement and select correct option

Physical Chemistry
General158 A Both cis 1 3 dimethyl cyclobutane and trans 1 3 dimethyl cyclobutane are optically inactive chy R cis 1 3 Dimethyl cyclobutane has the plane of symmetry whereas trans form has the centre of symmetry

Physical Chemistry
SolutionsWhich of the given observations are correct when a solution containing a non volatile solid solute is allowed to freeze i The vapor pressure of the solution is less than that of pure solvent ii The vapor pressure of the solution is greater than that of pure solvent iii Only solute molecules solidify at the freezing point iv Only solvent molecules solidify at the freezing point i iii i iv ii iii ii iv

Physical Chemistry
Gaseous and liquid states1000 mol of ammonia is isothermally compressed from 5 to 0 5 m at 300 K Calculate the work in this process if the change of pressure with volume obeys the following equation a P RT V b V m Note a 423 3 kPa and b 0 0373 m kmol kmo Answer W 5155 86 kJ solve it using the formula W integral of P dV from interval V to Vf

Physical Chemistry
Chemical BondingA reaction rate increases by a factor of 500 due to the presence of a catalyst at 37 C If the reaction is carried out in presence of a catalyst at 127 C by what factor compared to original rate in absence of catalyst at 27 C the rate will increase Activation energy of origina pathway is 106 kJ mol A 5 05 10 6 C 5 05 B 5 05 106 D 106

Physical Chemistry
EquilibriumSulfur and Fluorine react in a combination reaction to produce sulfur hexafluoride In a particular experiment the percent yield is 83 1 This means that in this experiment A 4 50 g sample of fluorine yields 828

Physical Chemistry
General23 4 milliliters of liquid nitrogen are allowed to turn boil into a gas at 298K If the density of liquid nitrogen is 0 808 g mL what is the volume of the nitrogen assuming that the pressure is 744 mmHg

Physical Chemistry
General69 Holme s signals can be obtained by using 2 CaC Ca P 4 Ca P CaCN 1 CaC CaCN 3 CaC CaCO Br 69 Holme s signal feh 4 2 CaC Ca P 4 Ca P CaCN aix re 1 CaC CaCN 3 CaC CaCO

Physical Chemistry
Chemical kineticsThe half life of a substance in a certain enzyme catalysed reaction is 138 s The time required fo the concentration of the substance to fall from 1 28 mg L 1 to 0 04 mg L is 1 690 s AIPMT Mains 201 2 276 s

Physical Chemistry
General3 Cr NH3 4 NO CO NH3 NO 4 4 Co NH3 5 NO Cr NH3 NO 5 51 Possible linkage isomers of the compound Co NH3 4 SCN NO 1 2 2 3 3 4 4 1 NO CO NH3 NO 5 2 Co NH3 6 Cr NO 6 X 3 Cr NH3 4 NO CO NH3 2 NO 4 4 Co NH 5 NO Cr NH3 NO 5 61 Co NH3 4 SCN NO affa OFF THE 1 2 S4 N 2 3 NCS 3 4 4 1 S CN NO 2