Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
EnergeticsFor the reaction of one mole of zinc dust with one mole of sulphuric acid in a bomb calorimeter AU and w correspond to a AU 0 w 0 c AU 0 w 0 b AU 0 w 0 d AU 0 w 0 2005 2015

Physical Chemistry
Equilibrium20 mL of 0 032 M AgNO3 are mixed with 15 0 mL of 0 041 M of NaBr at 25 C Ksp of AgBr 5 x 10 13 at 25 C Then O the precipitation of AgBr occurs as the solution will be supersaturated with AgBr AgBr will not be precipitated as the solution will be unsaturated the resulting solution will be just saturated with AgBr and no precipitation takes place no prediction can be done regarding the spontaneity of precipitation 0

Physical Chemistry
Atomic StructureWhich of the following energy change is less than third Balmer transition in He ion 1 First excitation energy of He ion 2 Third separation energy of Li ion 3 Fourth excitation energy of hydrogen atom

Physical Chemistry
Gaseous and liquid states23 Four one litre flasks are separately filled with the gases CO F2 NH3 and He at same room temperature and pressure The ratio of total number of atoms of these gases present in the different flasks would be 1 1 1 1 1 3 3 2 4 1 2 1 2 2 3 4 2 1 3 2 16 21 26

Physical Chemistry
General56 If activation energy of a reaction is 800 cal mol at temperature 200 K The percentage of molecules having energy greater or equal to E is Given e2 7 38 a 1 2 7 3 25 2 4 13 5 1 35

Physical Chemistry
Chemical kinetics5 For the reaction CH CI OH aq aq The kinetic data are as given below CH CI OH CH OH CT aq a 10 18 b 10 15 c 10 5 aq d CH OH dt M min 3 0 2 0 1 2 10 0 4 0 1 4 10 0 4 0 2 8 10 X If K for the above reaction is 1 10 4 then the specific reaction rate M min for the replacement of OH group of methanol by Cl atom is

Physical Chemistry
Energetics51 Which of the following expressions is true 1 1 A H CO 9 4 H CO 9 2 4 H CO g 4 H C graphite 4 H 0 9 1 3 A H CO g A H CO 9 4 H 0 9 2 4 AFHO CO g A combH C graphite Acomb H CO g 41 51 f Ah Het H10 W 90 which 2 1 K 2 Nie 1 AH CO 9 4 H CO 2 9 CH02 3 Bot 4 No 2 AH CO g A H C graphite 4 H which 1 Hig 2 3 A H CO 9 A H CO 9 A H H 3 ox 4 A H CO g A combH C graphite AcombH 4 A 1

Physical Chemistry
General151 A Complex Co en NO CI can exhible isomerism R Ethylene diamine en is bidentate ligand 152 A Fe CO complex is more stable than V CO R Fe CO obeys EAN rule but V CO does not N2 VO R Ethylene diamine en u fac fartus i 152 A Fe CO Highel V CO Highed at gaal f ch full R Fe CO Fight EAN 4 YIGH fch VCO 93 ATIMS PATTERN TEST 01 Date 30 10 2017 E 2 Lot

Physical Chemistry
General4 74 A 1 85 g sample of an arsenic containing pesticide was chemically converted to AsO 3 atomic mass of As 74 9 and titrated with Pb to form Pb ASO If 20 mL of 0 1 M Pb is required to reach the equivalence point the mass percentages of arsenic in the pesticide sample is closest to a 8 1 b 2 3 c 5 4 d 3 6

Physical Chemistry
Chemical kinetics1 2 32 Four vessels 1 2 3 and 4 contain respectively 10 mol atom t1 2 10 hours Imol atom t 5 hours 5 mol atom t 2 2 hour and 2 mol atom t 2 1 hour of different radioactive nuclides In the beginning the maximum radioactivity would be exhibited by the vessel a 4 b 3 c 2 d 1

Physical Chemistry
Nuclear chemistry1 Mole of an ideal gas initially at 400 K and 10 atm is first expanded at constant pressure till the volume is doubled Then the gas is made to undergo an isochoric process in which its temperature is found to decrease In the last final step gas compressed reversible and adiabatically to initial state Determine the net work involved in this cyclic process in terms of R Given Cv for gas 1 5 R 4 1 3 0 63 If W 2R xz report your answer as z

Physical Chemistry
General4 1380 g 4 1380 g 48 A compound is composed of 74 C 8 7 H and 17 3 N 48 74 C 8 7 H3 17 3 HR by mass If the molecular mass of the compound is 162 162 what is its molecular formula 1 C H N 10 14 2 C H 0 10 10 3 C H 4N 10 14 4 C H 4N 19 AU is equal to 2012 74 x 10 7 x 19 6 173 17 17 1 1 C 0H 4N 2 C H N 10 10 2 3 C H N 10 14 4 C H 4N 14 49 AU FRIOR

Physical Chemistry
Generalelect the net ionic equation for the reaction of hypochlorous acid HOCl aq with HaOH aq a H aq OCH aq Na aq OH aq H O 1 Na aq OCI aq b H aq OH aq H O 1 c HOCl aq Na aq OH aq OCT aq H O 1 Na aq HOCl aq OH aq OC aq H O 1 d

Physical Chemistry
GeneralOne mole triatomic vapours of an unknown substance effuses 4 3 times faster than 1 mol O under same conditions If the density of unknown vapours at pressure P and temperature T is d which of the following holds true for the unknown substance dN T P 0 8035 g Z atomic number 6 Z compress ibility factor 18P dRT Vapour density 9

Physical Chemistry
Atomic Structure128 The first orbital of H is represented by 3 2 4 1 T probability of finding the electron at a distance r from the nucleus in the region dV is a y dr u 4tr dr V y riao where ao is Bohr s radius The 27 2 e 9 2 b y 4 r dv d Sydv

Physical Chemistry
GeneralWhat is the total number and mass of neutrons in 7 mg of 14C Assume that mass of a neutron 1 675 x 10 27 kg 1 2 41 1021 4 03 10 6 kg 2 6 23 1023 1 67 107 3 1 22 1022 4 03 106 kg 4 2 41 x 1021 4 03 10 6 g 21 kg

Physical Chemistry
Equilibrium5 The heat of neutralization of a strong base and a strong acid is 57 kJ mol The heat released when 0 5 mole of HNO3 solution is added to 0 20 moles of NaOH solution is a 11 4 kJ b 34 7 kJ c 23 5 kJ d 58 8 kJ 2002

Physical Chemistry
Atomic Structure17 The frequency of first line of Balmer series in hydrogen atom is vo The frequency of corresponding line emitted by singly ionised helium atom is a 2v0 b 4 vo d vo 4 c vo 2 When atoms are bombarded with a particles only a fe

Physical Chemistry
General4 15 3 10 4 15 49 A gas expands from 3 dm to 5 dm against a constant 49 3 atm 3 dm 5 dm de pressure of 3 atm The work done during the expansion is used to heat 10 mol of water at temperature 290 K Find Yuraka f hell 290 the final temperature of water if the specific heat of water 4 18 Jg K 1 298 808 k 3 292 808 k 2 290 808 k 4 300 k 5 5 1 faf3 4 181 K 1 298 808 k 3 292 808 k Sy 3 M 2 290 808 k 4 300 k 4 Volume g H gas ST Hence work 1 22 4 L a 2 5 6 L at Idm33 11 2 L 11 4 44 8 L Given N g The sta

Physical Chemistry
EnergeticsAtomic Structure Chemical Arithmetics Solution Thermodynamics fe aliquise alegien gravfer Fe O s 3 H g 2 Fe s 3H O AHO 35 kJ 1 af fag f AHD 26 KJ TITUTE 65 For reduction of ferric oxide by hydrogen Fe O s 3 65 H g 2 Fe s 3H O AHO 300 35 kJ The reaction was found to be too exothermic To be convenient it is desirable that AHO should be at the most 26 kJ At what temperature is it possible C Fe O3 105 C Fe s 25 C H O 75 C H 9 29 all are in J mol 1 480 43 K 2 508 43 K 3 580 43 K 4 408 43 K plo of an idal gas A C 3R and 2 mole of an ideal 66 C3R 1 mole s 300 C Fe 0 105 C Fe s 25 CH O 0 75 CH Q 29 J mol 1 480 43 K 2 508 43 K 3 580 43 K 4 408 43 K

Physical Chemistry
Gaseous and liquid states4 One mole of ideal gas goes through process P 2V2 1 V2 Pa then change in temperature of gas when volume changes from V 1m to 2 m is 1 3 4 5R 5 K K 2R 11 2 5R 4 2K K

Physical Chemistry
EquilibriumThe frequency v of certain line of the Lyman series of the atomic spectrum of hydrogen satisfies the following conditions i It is the sum of the frequencies of another Lyman line and a Balmer line ii It is the sum of the frequencies of a certain line a Lyman line and a Paschen line

Physical Chemistry
Solid stateSolveLancer Test For which lattice system all 4 primitive face centered side centere and body centered lattice types are observed SolveLancer Test a Cubic b Orthorhombic c Tetragonal d Triclinic

Physical Chemistry
SolutionsIn the saturated aqueous solution of PbC1 the freezing point of water decreases by DC 100 then D is Given k of PbCl 4 x 106 Kwater 2 K kg mole assume molality molarity A roversible evelie process involves 6 etong In eten 1 and 2 motom absorb 500 Land 800 I of

Physical Chemistry
ElectrochemistryA hydrogen gas electrode is made by dipping platinum wire in a solution of HCl of pH 10 and by passing hydrogen gas around the platinum wire at one atm pressure The oxidation potential of electrode would be a 0 118 V c 0 059 V b 1 18 V d 0 59 V NEET 2013

Physical Chemistry
Equilibrium58 For a reaction A g B g at equilibrium The partial 68 pressure of B is found to be one fourth of the partial pres sure of A The value of AGO of the reaction A B is 1 RT en 4 3 RT log 4 2 RT en 4 4 RT log 4 4 3C H 9 C H C A g B Rich D A Rich and ens Juri Giala AB 1 RT en 4 3 RT log 4 g AGA 2 RT en 4 4 RT log 4 Torrent Q R T kilo

Physical Chemistry
GeneralThe product of following reaction will be Br OH Br Br Br H 0 Br Br 8 8 OH Product Br OH Br OH OH Br OH An electric discharge is passed through a mixture 77 78 79 1 For WH K 3 Wh pho 1 3 Ho are Cor
Physical Chemistry
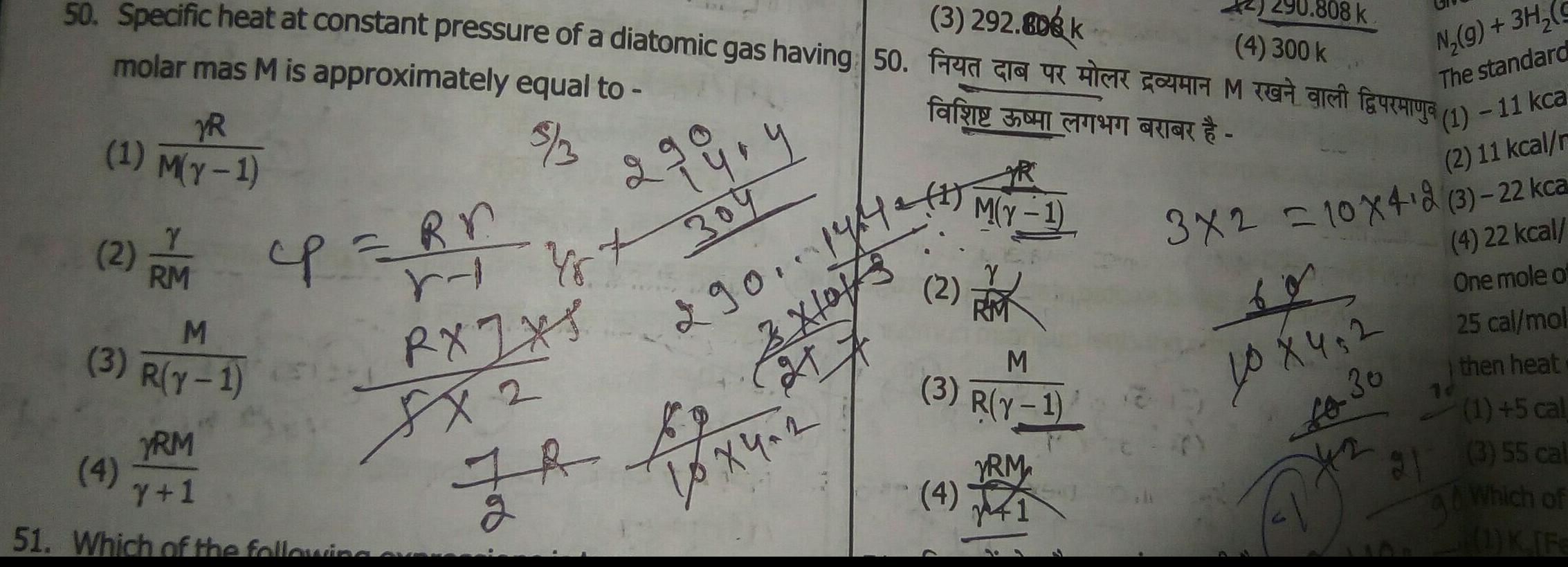
General3 292 808 k 4 300 k 50 Specific heat at constant pressure of a diatomic gas having 50 FK COM O faucurya molar mas M is approximately equal to far CRICK 3 3 YR 1 M y 1 Y 2 2M cp Rr RM M 3 R Y 1 RA 8x2 YRM 4 Y 1 51 Which of the following 2 99 4 304 290 1942 Xlo r Yst JR By 27 2 10x4 2 2 M Y 1 M 3 R Y 1 YRM 4 41 808 k N g 3H g The standard 1 11 kca 2 11 kcal r 3x2 10x4 2 3 22 kca 4 22 kcal One mole of 25 cal mol then heat 1 5 cal 31 3 55 cal Which of 1 K Fe 40 X 4 2 Lo 30 A

Physical Chemistry
Solutions58 59 1 17 g of NaCl in one litre water If density of 2 molal sucrose solution is 1 4 g mL at 25 C find osmotic pressure 1 4 06 atm 2 2 atm 3 40 6 atm 4 3 4 atm How many unit cells of KBr are present in Imm 66 in nu 1 3 Flu U

Physical Chemistry
EquilibriumAIIMS The charge balance equation of species in 0 100 M acetic acid solution is given by a H OH c H OH CH COO d 2 H QH ICH COO b H CH COO

Physical Chemistry
GeneralZero Marks 0 In all other cases Assume isotope of chlorine present on the unknown planet are 34C1 and 38 Cl If average molecular weight of Cl is found to be 35 What is the sum of moles of proton and neutron in 7 gm of sample of chlorine

Physical Chemistry
Energetics8 For a given reaction energy of activation for forward E is 80 kJmol 1 reaction AH 40 kJmol 1 for the reaction A catalyst lowers E by 20 kJ mol 1 The ratio of energy of activation for reverse reaction before and after addition of catalyst is 1 1 0 Quin 2 0 5 prib1000A 3 1 2 tspol att bido 4 2 0 nitido ad

Physical Chemistry
GeneralOne mole of an ideal gas is allowed to expand freely and adiabatically into vacuum until its volume has doubled A statement which is not true concerning this expression is 1 a c AH 0 b AS 0 AE 0 d W 0

Physical Chemistry
EquilibriumWhich of the following is a buffer solution Question Type Single Correct Type 1 2 3 500 ml of 0 1 N CH3COOH 500 mL of 0 1 N NaOH 500 ml of 0 1 N CH3COOH 500 mL of 0 1 N HCI 500 ml of 0 1 N CH3COOH 500 mL of 0 2 N NaOH 500 ml of 0 2 N CH3COOH 500

Physical Chemistry
General5 24 256M NITE NA D 0 089 Vapour density of a gas if its density is 0 178 g L at NTP is A 0 178 B 2 C 4 In an organic compound of molar mass greater than 100 containing only C H and N the percentage of

Physical Chemistry
GeneralA 2 00g serving of uni contains 7 2 ug essential nutrient compound if the compound is 22 2177 nitrogen by mass and one molecule of this compound contains 7 nitrogen atoms which is the molar mass of this nutrient in g mol

Physical Chemistry
GeneralWhat is the enthalpy change in kJ of a chemical reaction that raises the temperature of 250 0 ml of solution having a density of 1 25 g mL by 3 33 OC The specific heat of the solution is 3 74 J g K Select one a 6 51 b 7 43 c 8 20 d 3 89 X e 3 89

Physical Chemistry
Atomic Structure4 All of these are correct 65 The wave number of electromagnetic radiation emitted during the transition of electron in between two levels of Li ion whose principal quantum numbers sum is 4 and difference is 2 is 1 R 2 4R 3 8R 8 4 R

Physical Chemistry
Chemical BondingWhen heat is absorbed then solution of enthalpy is positive then bond will not dissociated and when heat is released then negative so bond will dissociated Am I right solution of enthalpy is 5 56 AM

Physical Chemistry
General22 0 078 gms of a gaseous hydrocarbon occupy 44 8 ml volume at 1 atm and 273 C The empirical formula of the hydrocarbon is CH Find total number of atoms in one the molecule of the hydrocarbon Chenen STI

Physical Chemistry
General63 For the process H O atmosphere pressure the correct choice is 1 AS 0 and AS 0 system 0 0 3 AS w BSE H O g at T 100 C and 1 63 H O l H O g 100 C fouT 4 AS system system surroundings 2 AS 0 and AS system 0 and AS 0 and AS DSPARTY surroundings 0 4 A sample of liquid in a thermally insulated container 64 3 surroundings DS fly surroundings DSURG 2120 1 AS 0 and AS Fight 2 AS Fach 3 AS Fac 0 and AS 0 Ra Ra 0 and AS 4 AS 0 and AS fa 0 0 0 1

Physical Chemistry
Solid stateIn the close packed structure of AB type solid have cation radius of 75 pm what would be the maximum and minimum sizes of the anions which formed voids in unit cell A minmum 101 45 r maximum 303 3 pm B r minmum 105 45 r maximum 300 3 pm C r minmum 102 45 r maximum 333 3 pm 333 3 pm minmum 98 4 r maximum

Physical Chemistry
Gaseous and liquid statesA gas filled weather balloon with a volume of 65 0 L is held at ground level at an atmospheric pressure of 732 mmHg and a temperature of 22 0 C The balloon is released and rises to about 18 000 m where the temperature is 4 35 C and the balloon has expanded to 852 L Calculate the pressure at the higher altitude a 61 288 torr b 61 3 mmHg c 50 885 mmHg d 10530 torr e 50 9 torr

Physical Chemistry
Generalrule Dau s ty of nt of is m m 73 For an atom or ion having single electron compare the energies of the following orbitals S a spherical symmetrical orbital having two spherical nodes S an orbital which is double dumb bell and has no radial node S an orbital with orbital angular momentum zero and three radial nodes S an orbital having one angular and one radial node 1 S S S S 3 S S S S 2 S S S S 4 S S S S

Physical Chemistry
Electrochemistry1 61 For the cell H pH 1 H pH 2 H 0 1 atm Pt Pt H 0 4atm the measured potential at 25 C is 1 0 025 V 2 0 76 V 3 0 041 V 4 None 62 When H reacts with Na it acts as a 800 700 1500 avo oo 61 fu Pt H 0 4atm H pH 1 H pH 2 H 0 1 atm Pt 25 C 1 0 025 V 3 0 041 V TUELT 2 0 76 V 4 None 62 H H a 2 la 103 ph day 1

Physical Chemistry
Atomic StructureEmission transitions in the Paschen series end at orbit n 3 and start from orbit n and can be represented as v 3 29 x 1015 Hz 1 32 1 n Calculate the value of n if the transition is observed at 1285 nm 1 6 2 5 3 8 4 9

Physical Chemistry
General4 1 1 g sample of copper ore is dissolved and Cu aq is treated with KI The I so liberated required 12 12 mL of 0 1 M Na S O3 solution for titration What is of Cu in the ore

Physical Chemistry
Surface chemistryIn coagulation of a gold sol which of the following Fe CN 6 4 PO4 3 SO4 2 CI will have 1 Greatest flocculation value 2 Greatest coagulation value 3 Greatest coagulation power 4 Greatest flocculation power
Physical Chemistry
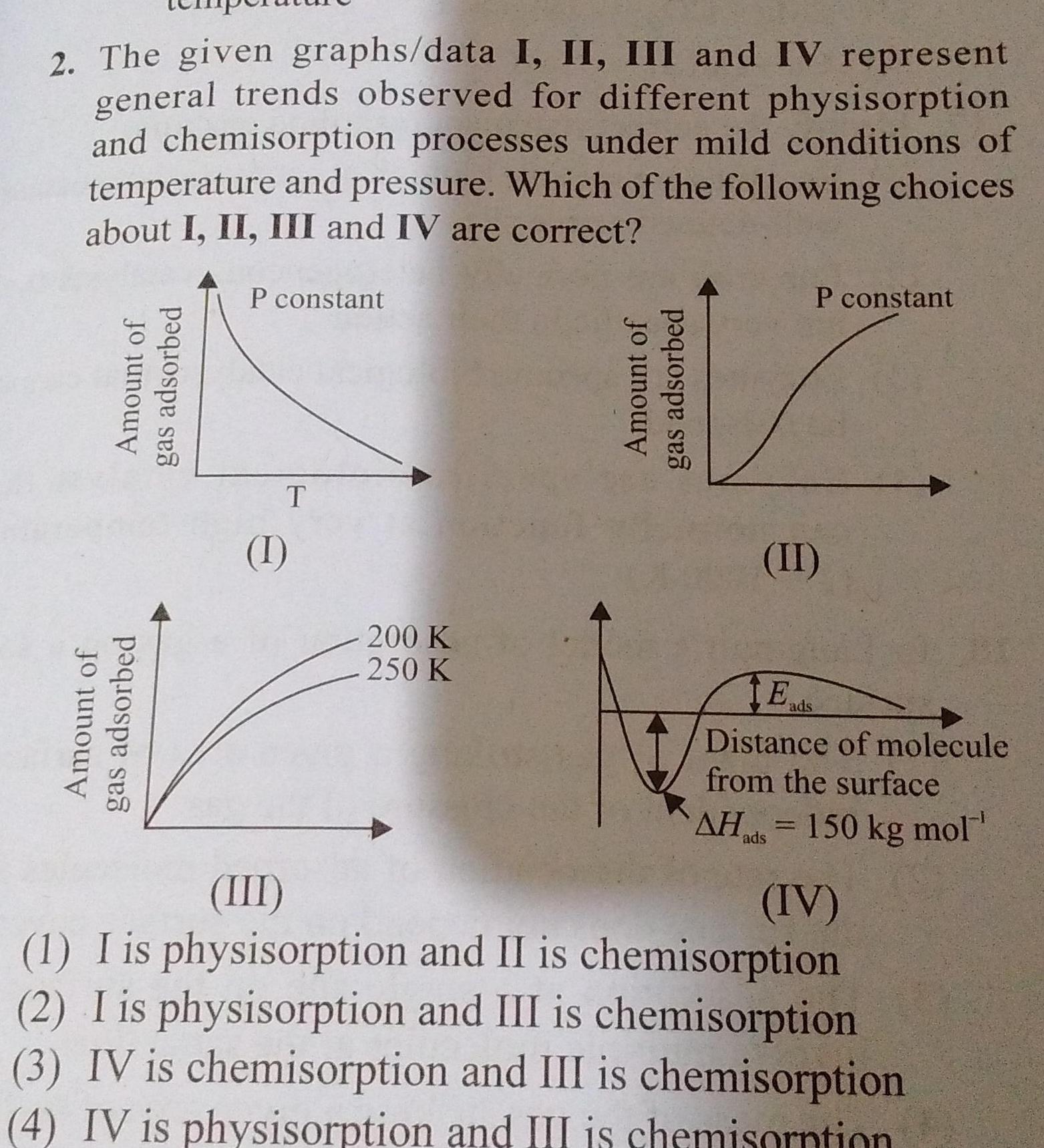
Surface chemistry2 The given graphs data I II III and IV represent general trends observed for different physisorption and chemisorption processes under mild conditions of temperature and pressure Which of the following choices about I II III and IV are correct Amount of gas adsorbed Amount of gas adsorbed P constant T 1 200 K 250 K Amount of gas adsorbed P constant II E Distance of molecule from the surface AHads 150 kg mol IV ads III 1 I is physisorption and II is chemisorption 2 I is physisorption and III is chemisorption 3 IV is chemisorption and III is chemisorption 4 IV is physisorption and III is chemisorption

Physical Chemistry
GeneralNegative Marks 1 If wrong option is selected The degree of dissociation of A is 10 then find molecular weight of A for given reaction when vapour density of mixture is 60 A g B g C g O 120 O132 O 60 O 40