Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Chemical kineticsConsider the following graph for the reaction A P AT 2 Lmol 8 tan 0 5 0 0 t min choose the correct statement s The given reaction is a second order reaction The given reaction is a third order reaction O Initial half life of reaction is 4 min Initial concentration of A is 2M

Physical Chemistry
GeneralThe partial pressure of ethane over a solution containing 6 56 103 g of ethane is 1 bar If the solution contains 5 00 10 2 g of ethane then what shall be the partial pressure of the gas Delhi 2013 C AI 2012 C

Physical Chemistry
Chemical kineticsQ 23 O 30s For a I order reaction A B the reaction rate at reactant concentration 0 01 M is found to be 2 0 10 Ms The half life period of the reaction is O 300s 4 1 C 00 05 220s O 000

Physical Chemistry
Energetics24 Bond energies in kcal mol of different types of bonds have been given as C C bond 40 H H 104 and C H 87 c c C C H H cc HH Heat of hydrogenation of the above reaction is a 57 kcal mol b 57 kcal mol c 30 kcal mol d 30 kcal mol

Physical Chemistry
Chemical kineticsQ 3 O 0 04 DERATE 0 8 4 1 00 35 The rate constant for the reaction 2 N O 4NO O is 3 0 x 10 s If the rate is 2 4 x 105 mol L s then the concentration of N O in mol L is O

Physical Chemistry
General7 The centre of mass of a system of particles does not depend on 1 Position of the particles 2 Relative distances between the particles 3 Masses of the particles 4 Forces acting on the particles

Physical Chemistry
GeneralQ 10 moderate 4 1 00 20 Alkaline earth metals are not found free in nature because of O Their high b p O Their low b p Thermal instability 7 Their great chemical

Physical Chemistry
Surface chemistryQ 11 On adding 1 mL solution of 10 NaCl to 10 mL gold solution in the presence of 0 25 g of starch the coagulation is just prevented Starch has the gold number equal to O 0 25 O 2 5 4 1 00 33 250 BEL

Physical Chemistry
General220 45 The quantum numbers of four electrons are as follows n 0 Electron 1 3 1 Electron 2 3 Electron 3 4 iv Electron 4 3 0 2 1 0 0 2 46 fra t S NI NIH NI NIN Batch Chemical Arithmetic Atomic Structure Solid State 2 1 Electron 1 3 n Electron 2 3 ii Electron 3 4 iv Electron 4 3 0 0 0 Nie nie nie wie

Physical Chemistry
GeneralQ 3 4 1 00 19 Addition of FeCl to K Fe CN in dilute and cold solution gives O Prussian blue sol O Fe Fe CN Sol O Positive sol C

Physical Chemistry
Chemical kinetics4 A catalyst lowers the activation energy of a certain reaction at 27 C from 75 to 29 kJ mole If other parameters are same rate constant of the reaction 1 Increases by 108 times 2 Decreases by 104 times 3 Increases by 104 times 4 Decreases by 108 times

Physical Chemistry
Surface chemistryDuring adsorption on a solid diatomic molecules of a gas X dissociate into atoms Which of the following relationships are true for the said process 0 surface coverage on a uniform adsorbent surface ka is the rate constant of adsorption and ka is the rate constant of desorption P is the pressure of gas X 8 KP 1 KP KPZ 1 1 KPZ K K K

Physical Chemistry
Chemical kineticsQ 21 4 00 17 The time for half life period of a creation reaction A products is 1 h when the initial concentration of the reactant A is 2 0 molL how much time 4h does it take for its concentration to come from 0 50 to 0 25 mol L if it is a zero order reaction 1

Physical Chemistry
EquilibriumGurukripa CAREER INSTITUTE 73 For the process H O 1 1 bar 373 K H O g 73 HH O 1 bar 373 K H O g 1 bar 373 K 1 bar 373 K the correct set of thermodynamic parameters is 1 AG ve AS 0 2 AG 0 AS ve 3 AG 0 AS ve 4 AG ve AS 0 4 An anion X has 31 nucleons and has atomic number equal 74 in it is 1 AG ve AS 0 2 AG 0 AS ve 3 AG 0 AS ve 4 AG ve AS 0 X 331 THYHE and de

Physical Chemistry
Generaloblem 3 1 08 g of copper wire was allowed to react with nitric acid The resulting solution was dried and ignited when 1 35 g of copper oxide was obtained In another experiment 2 30 g of copper oxide was heated in presence of hydrogen yielding 1 84 g of copper Show that the above data are in accordance with law of constant proportion

Physical Chemistry
GeneralFor the two moving particles A and B if the momentum of particle A having wavelength 1 is double of particle B ther the wavelength of particle B is 2 0 5 4

Physical Chemistry
Solutions27 How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2 0M HNO3 The concentrated acid is 70 HNO3 2013 a 90 0 g conc HNO3 b 70 0 g conc HNO3 c 54 0 g conc HNO3 450 g conc HNO

Physical Chemistry
Chemical BondingA gaseous mixture of three gases A B and C has a pressure of 10 atm The total number of moles of all the gases is 10 If the partial pressure of A and B are 3 0 and 1 0 atm respectively and if C has mol wt of 2 0 what is the weight of C in g present in the mixture

Physical Chemistry
Solutions0 A For an ideal binary liquid solution with P Po which relation between X mole fraction of A in liquid phase and YA mole fraction of A in vapour phase is correct YA YB XA XB YAYB XA XB Y Nos X XD

Physical Chemistry
GeneralD Consider the reaction 2x g 3y g 2z g At constant temperature and volume partial pressure of x p varies as follows Time min 0 100 200 Order of the reaction is 1 O 3 1 px mm Hg 800 400 200 2 0 5 4 2

Physical Chemistry
Chemical kineticsQ 7 4 1 O 72 times O 8 times 00 11 The reaction 2A B CD E is found to be first order in A second in B and zero order in C What is the effect on the rate of increasing concentration of A B and C two times 24 times O

Physical Chemistry
GeneralQ 31 For the Ch the initial concentration of was 0 20 mol L and the concentration after 20 min was 0 18 mol L Then the rate of formation of 1 in mol L would be 4 1 00 43 reaction 21 12 2 CF 1810 4

Physical Chemistry
GeneralIf decomposition reaction A g B g follows first order kinetics then the graph of rate of formation R of B against time t will be KU 2 1 3 14 4 h Decomposition of NH NO aq into N g and 2H O 1 is first order reaction Which of the following graph is correct 1 0 288 2 0 577 3 1 154 4 none If above where you

Physical Chemistry
GeneralThe standard reduction potential of normal calomel electrode and reduction potential of saturated calomel electrodes are 0 27 and 0 33 volt respectively What is the concentration of CI in saturated solution of KCI 1 0 1 M 2 0 01 M 3 0 001 M 4 None

Physical Chemistry
General3 85 The value of AH in kJ for the reaction will be 6 2 6 2 4 24 2 2 CS 1 4NOCI g CCI 1 2SO g 2N g if AHO NOCI y AHO SO r An AHO CS x X AH CC Z 1 x 4y z 2r 2 r z 4y x 3 2r z 4y x 4 x 4y z 2r K 2 6 4 5 3 15 85 3K 1 CS 1 4NOCI g CCI 1 25O g 2N g AHO CS x AHO NOCI Y AHO CCI z AHO SO r 1 x 4y z 2r 2 r z 4y x 3 2r z 4y x 4 x 4y z 2r

Physical Chemistry
GeneralWhich of the following statements is not correct O a Standard deviation is a measure of precision b All types of equilibrium are dynamic OC Percent dissociation of HF increases with increasing its concentratic d Buffer activity is most active at pH pkc

Physical Chemistry
Nuclear chemistryQ 14 O 10 4 1 O 5 10 4 00 19 The rate constant of a first order reaction at 27 C is 10 min The temperature coefficient of this reaction is 2 What is the rate constant in min at 17 C for this reaction 000

Physical Chemistry
Chemical kineticsQ 13 04 Half life of a reaction is found to be inversely proportional to the cube of initial concentration The order of reaction is 03 4 85 1 00 10 388

Physical Chemistry
GeneralBombardment on nucleus A by a particle leads to its artificial disintegration in two ways i and ii as shown Products X Y and Z respectively are Oproton neutron positron O neutron positron proton Oproton positron neutron Opositron proton neutron 27 13 i 30 14B X 30 15 C Y 30 14D Z

Physical Chemistry
General5 The chromium in a 1 0 g sample of chromite FeCr2O4 was oxidized to Cr 6 state by fusion with Na2O2 The fused mixture was treated with excess of water and boiled to destroy the excess of peroxide After acidification the sample was treated with 50 mL of 0 16 M FeSO4 solution 3 67 mL of 0 05 M Cr O solution was required to oxidize the Fe2 ion left unreacted Determine the mass of chromite in the original sample Cr 52 Fe 56

Physical Chemistry
GeneralQ 19 O 0 5 0 O 1 250 4 O 12 5 M 1 K for a zero order reaction is 2 102 mol L sec If the concentration of the reactant after 25 sec is 0 5 M the initial concentration must have been 00 31 000

Physical Chemistry
Gaseous and liquid statesPROBLEM 42 A careless student while determining the molecular weight of chloroform CHC13 found that 0 1008 g of the liquid displaced 19 00 mL of air at 16 5 C and 707 5 mm pressure Calculate the percentage error aq tension at 16 5 C 13 5 mm Ans 15 5

Physical Chemistry
GeneralKMnO is a good oxidising agent and commonly used to estimate many compounds 400 m KMnO solution was divided in two parts 3 4 part of solution was completely reacted w 200 ml of H O solution and remaining 1 4 part was completely reacted with 200 ml of 50 H C O solution both in acidic medium X If molarity of KMnO is X then find the value of

Physical Chemistry
Chemical kineticsQ 5 4 1 Half life period of a first order reaction is 1386 seconds The specific rate constant of the reaction is O 5 0 10 00 16 05 0 10 s O 0 5 10

Physical Chemistry
Solid statetod Vin 1 4000 Pa 2 2000 Pa 3 1000 Pa 4 500 Pa Yor 4 500 Pa 4 An alloy on Zn and Cu i e brass weighs 16 8g in air 44 Zn and 14 7g in water If relative density of Cu and Zn are 8 9 and 7 1 respectively then determine the amount of Zn and Cu in the alloy 1 8 8 g 8 g 2 8 4 g 8 4 g 3 9 4 g 7 4 g 4 9 9 g 5 9 g Ice pieces are fir 10x1 5X1 4 4 14 7g 1 fe 7 1 CHE muy m n 16188 1 8 8 9 8 g Light 2 8 4 g 8 4 g 3 9 4 g 7 4 g 4 9 9 g Ve 211 my

Physical Chemistry
GeneralA sample of hydrogen peroxide solution has a mass of 4 599 g After reacting with the enzyme solution the moles of oxygen are 0 00284 moles What is the percent hydrogen peroxide in the solution

Physical Chemistry
Energeticsa afe 1 AH is ve AS is ve AH 2 AH is ve AS is ve 3 AH is ve AS is ve 4 AH is ve AS is ve 3 AH is ve AS is ve 4 AHTS ve AS is ve 17 The enthalpy change of which reaction corresponds to 47 for fun after AH for Na Co s at 298 K AH fe but AH TAS 3 1 2Na s C s O g Na CO s 2 Na O s CO g Na CO s 3 2Na aq CO aq Na CO s 4 2Na aq 2OH aq CO aq Na CO s H O Which of the following is 3 112Na s C s O g 2 Na O s CO g Na Cr 3 2Na aq CO aq 0 300

Physical Chemistry
General1 A mixture of NH4NO3 and NH4 2HPO4 contain 30 40 mass per cent of nitrogen What is the mass ratio of the two components in the mixture 1 2 1 2 1 2 3 3 4 4 4 1 2 A metal M forms the sulphate M SO4 3 A 0 596 gram sample of the sulphate reacts with excess BaCl to give 1 220 g BaSO4 What is the atomic weight of M Atomic weights S 32 Ba 137 3 1 26 9 2 69 7 3 55 8 4 23 3 SO Cl sulphuryl chloride reacts with water to given a mixture of H SO4 and HCl What volume of 0 2 M Ba OH is needed to completely neutralize 25 mL of 0 2 M SO Cl solution 1 25 mL 2 50 mL 3 100 mL 4 200 mL 100 cm of a solution of an acid Molar mass 98 containing 29 4 g of the acid per litre were completely neutralized by 90 0 cm of aq NaOH containing 20 g of NaOH per 500 cm The basicity of the acid is 1 3 2 2 3 1 4 data insufficient 5 A gaseous compound is composed of 85 7 by mass carbon and 14 3 by mass hydrogen It s density is 2 28 g litre at 300 K and 1 0 atm pressure Determine the molecular formula of the compound 1 C H 2 C H4 3 C4H8 4 C4H10 6 When 22 4L of H g is mixed with 11 2L of Cl g each at STP the moles of HCl g formed is equal to a 1 mole of HCl g b 2 moles of HCl g c 0 5 mole of HCl g d 1 5 moles of HCl g 7 1 c c of N 0 at NTP contains 1 10 2 atoms 6 02 x 1023 molecules 22400 3 10 electrons 4 All the above 8 What is the number of moles of O atom in 126 amu of HNO3 1 2 2 3 6 6 The number of gram molecules of oxygen in 6 02 102 4 CO molecules is 1 10 gm molecules 2 5 gm molecules 3 1 gm molecules 4 0 5 gm molecules 10 The haemoglobin from the red blood corpuscles of most mammals contains approximately 0 33 of
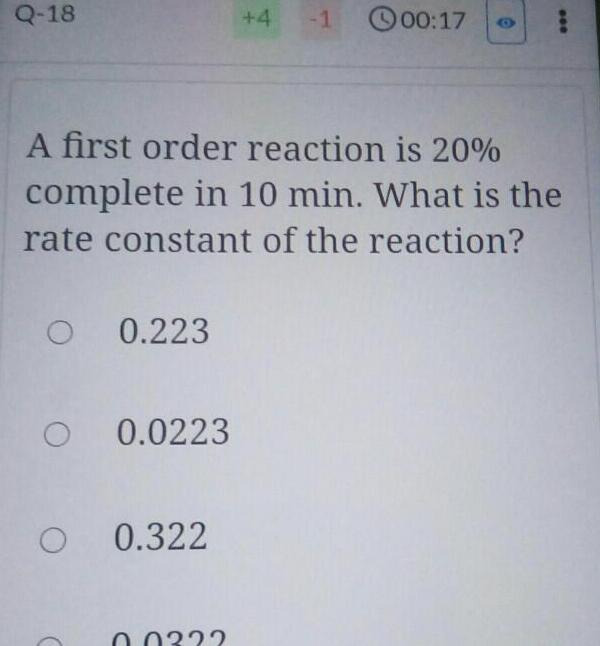
Physical Chemistry
Chemical kineticsQ 18 O 0 223 O 0 0223 O 0 322 4 A first order reaction is 20 complete in 10 min What is the rate constant of the reaction 0 0332 1 00 17 000

Physical Chemistry
Energetics4 90 For given following equations and AH values determine 90 the enthalpy of reaction at 298 K for the reaction C H g 6F g 2CF4 g 4HF g H g F g 2HF g AH 537 kJ C s 2F g CF4 9 AH2 680 kJ 2C s 2H g C H 9 AH 52 kJ 1 1165 2 2486 3 1165 4 2486 hld HR 9 12 fuff 298 K C H g 6F g 2CF g 4HF g H g F g 2HF g AH 537 kJ C s 2F g CF 9 AH 680 kJ 2C s 2H g CH g AH 52 kJ 1 1165 2 2486 3 1165 4 2486

Physical Chemistry
Chemical kineticsQ 8 O 8 min A reaction proceeds by first order 75 of this reaction was completed in 32 min the time required for 50 completion is O 16 min 4 1 00 23 O 20 min O 000

Physical Chemistry
Chemical kineticsQ 6 RATE 4 O 6 93 10 min The half life period of a first order reaction is 1 min 40 s Calculate its rate constant 6 93 10 5 1 06 93 10 s 00 14

Physical Chemistry
General60 A schematic representation of enthalpy changes for the 60 1 C graphite O g CO g reaction is given below The 2 missing value is Cgraphite O g 393 5 KJ 1 10 5 kJ 2 11 5 kJ 3 110 5 kJ 4 10 5 J CO g CO g 1 O g 283 0kJ CA C graphite f fi fu Cgraphite O g 393 5 KJ 1 10 5 kJ 2 11 5 kJ 3 110 5 kJ 4 10 5 J 1 0 g CO g fay tri urada f CO g 2 CO g 1 O g 283 0kJ 153 15ml f

Physical Chemistry
Generalc The standard enthalpy of formation of O g is 249 kJ mol Calculate the wavelength in nm of the electromagnetic radiation with the minimum amoun of energy required for dissociation of one molecule of oxygen gas 9

Physical Chemistry
Chemical kineticsQ 34 4 1 The half life of two samples is 0 1 and 0 4 s Their reactive O 0 00 30 concentration is 200 and 50 respectively What is the order of reaction 02

Physical Chemistry
Atomic Structurec excited state of Mg 9 Ionisation potential of hydrogen atom is 13 6 eV Hydrogen atom in the ground state are excited by monochromatic light of energy 12 1 eV The spectral lines emitted by hydrogen according to Bohr s theory will be CBSE PMT 1992 M13 a one three b two d four 3 wz 2 131

Physical Chemistry
EnergeticsPart III Calculating the Heat Associated with Phase Changes Physical Constants for Acetone Melting Point 94 7 C Boiling Point 56 1 C AHvap 31 3 kJ mol AH 5 7 kJ mol Molar Heat Capacity of liquid acetone is 125 J mol K Molar Heat Capacity of solid acetone is 96 J mol K 1 Calculate the energy required to heat 30 g of solid acetone C H O from 100 0 C to 42 0 C 2 Is heat released or absorbed in this process How can you tell 3 How would the amount of heat associated with this process change if only 15 grams was

Physical Chemistry
Surface chemistryWhich one of the following does not involve coagulation O Treatment of drinking water by potash alum O Formation of delta regions Peptization Clotting of blood by the use of ferric chloride

Physical Chemistry
Atomic Structure4 Can t be determined 4 Hond 6 8 eV 56 If the P E of an electron is 6 8 eV in hydrogen atom then 56 ISSIGA TRHY UN SAP E what is the K E total energy and the orbit where electron 3 K E exist are respectively 1 K E 6 8 eV Total energy 6 8 eV n 2 2 K E 3 4 eV Total energy 3 4 eV n 2 3 K E 3 4 eV Total energy 3 4 eV n 4 4 None of these 1 K E 6 8 eV 2 K E 3 4 eV 3 K E 3 4 eV 4 3 6 8 eV n 2 3 3 4 eV n 2 f 3 4 eV n 4 D L

Physical Chemistry
Electrochemistry3 4th 49 Bet and a proton are accelerated by the same potential 49 Be 3 fay CREER 4 3rd their de Broglie wavelengths have the ratio 1 1 2 2 1 4 3 1 1 4 1 3 3 Libe and 1 1 2 2 1 4 3 1 1 4 1 3