Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Chemical Bondingen C Which of the following statements is not correct regarding NO2 molecule 88 SCQ A Paramagnetic behaviour decreases when it undergoes in dimerisation B It is colourless in its dimeric form Correct Answer It can t follow octete rule in it s demeric form

Physical Chemistry
Gaseous and liquid statesA gas vessel is attached to an open end manometer filled with a non volatile liquid of density 0 993 g mL as shown below The difference in heights of the liquid in the two sides of the manometer is 32 3 mm when the atmospheric pressure is 765 mm Hg Given that the density of mercury is 13 6 g mL the pressure of the enclosed gas is atm Select one a 1 04 X b 0 993 c 1 05 d 1 08 e 1 01

Physical Chemistry
General27 A 25 0 mm x 40 0 mm piece of gold foil is 0 25 mm thick The density of gold is 19 32 g cm How many gold atoms are in the sheet Atomic weight Au 197 0 b 1 5x 10 23 c 4 3 1021 23 a 7 7 10 d 1 47 x 1022

Physical Chemistry
GeneralThe correct order of viscosity of ethanol ethylene glycol and glycerol is A Ethanol Ethylene Glycol Glycerol B Ethanol Glycerol Ethyleneglycol C Ethylene Glycol Ethanol Glycerol Correct Answer D Glycerol Ethylene Glycol Bl

Physical Chemistry
GeneralA concentration cell is a galvanic cell in which O The electrode material and the solution are made up of same substances but electrode and or electrolyte concentration are different Each half cell is made of electrode material and solutions that are composed of different substances and different concentrations Ecell 0 The nernst equation reduces to F 2 303 RT

Physical Chemistry
Electrochemistry4 An electro chemical cell is constructed by immersing a piece of Cu wire in CuSO solution and Zn strip in ZnSO4 solution The emf of cell increases when small amount of concentrated NH3 is added to Given Ecu u 0 34V E 0 76V 10 Zn 2 Zn 1 CUSO Solution 3 Both 1 and 2 2 ZnSO Solution 4 Can t be predicted

Physical Chemistry
GeneralQ 17 The rate constant for the first order reaction is 60s How O4 6 10 s 4 1 00 28 much time will it take to reduce the concentration of the reaction to 1 16 M value 4 6 10 s 4 6 x 10 s

Physical Chemistry
General15 mg radioactive sample 90 disintegrate in 4 years 99 9 disintegration will be in 90 afea atar m 15 mg af at 99 9 faufa A 4 years 4 f B 8 years 8 d C 12 years 12 ad D 16 years 16 Your Answer B

Physical Chemistry
Atomic StructureFor particles having same kinetic energy the de broglie wavelength Directly proportional to mass Inversely proportional to speed Inversely proportional to square root of mass Inversely proportional to square root of speed

Physical Chemistry
GeneralQ 9 The mass of carbon anode consumed giving only carbon dioxide in the production of 270 kg of aluminium metal from bauxite by the hall process is Atomic mass of Al 27 O 180kg 4 1 00 24 270 kg

Physical Chemistry
General11 Why is HCI not used to make the medium acidic in oxidation reactions of KMnO4 in acidic medium 1 Both HCI and KMnO4 act as oxidising agents 2 KMnO4 oxidises HCl into Cl which is also an oxidising agent 3 KMnO4 is a weaker oxidising agent than HCL 4 KMnO4 acts as a reducing agent in the presence of HCI

Physical Chemistry
Nuclear chemistryNitrogen occurs in nature in the form of two isotopes with atomic mass 14 u and 15 u respectively If the average atomic mass of nitrogen is 14 0067 u what is the abundance of the N 15 isotopes upto 2 decimals A 0 97 B C D 0 67 0 10 0 50

Physical Chemistry
Chemical Bondingen C Which of the following is not correct 73 SCQ A CH4CO2 order of EN of C atom B In Tl order of EN C NH3 PH3 ASH3 SbH3 lewis basic strength Correct Answer D NO Na20 Al2O3 acidic strength

Physical Chemistry
General5 In a saturated solution of the sparingly soluble strong electrolyte AgIO molecular mass 283 the equilibrium which sets in is AgIO3 s Ag aq IO3 aq If the solubility product constant Ksp of AgIO3 at a given temperature is 1 0 108 what is the mass of AgIO contained in 100 mL of its saturated solution 1 28 3 10 3 1 0 107 g 2 28 3 10 3 g 4 1 0 104 g

Physical Chemistry
Chemical kinetics55 It takes 1h for a first order reaction to go to 50 completion The total time required for the same reaction to reach 87 5 completion will be a 1 75 h b 6 00 h c 3 50 h d 3 00 h

Physical Chemistry
Energetics3 100 mL of 0 05 M CuSO4 aq solution was electrolyzed using inert electrodes by passing current till the pH of the resulting solution was 2 The solution after electrolysis was neutralized and then treated with excess KI and formed 1 titrated with 0 04 M Na S O3 Calculate the required volume in mL of Na S O3 1 112 5 mL 2 100 mL 3 125 ml 4 None of these

Physical Chemistry
GeneralThe most abundant elements by mass in the body of a healthy human adult are Oxygen 61 4 Carbon 22 9 Hydrogen 10 0 and Nitrogen 2 6 The weight which a 75 kg person atoms are replaced would gain if all 1 by 2H atoms is A 7 5 kg B 10 kg C c 15 kg D 37 5 kg H

Physical Chemistry
GeneralQ 37 The reaction 25O g O g 250 g is carried out in a 1dm vessel and 2d m vessel separately The ratio of the reaction velocities will be O 1 8 O 1 4 C 4 1 00 19 4 1

Physical Chemistry
ElectrochemistryNernst equation is curve is 1 2 3 E E LAY E E E E A Ant 0 059 n EA A 0 0 A 1 log A EAA 0 0 A 1 log A log A 0 0 A 1 A A log A ENA 0 A 1

Physical Chemistry
GeneralThe rate constant of the reaction A B is 0 6 10 3 mole per second If the concentration of A is 5 M then concentration of B after 20 minutes is 2015 RS a 1 08 M c 0 36 M b 3 60 M d 0 72 M

Physical Chemistry
Equilibrium12 Calculate the amount of NH4 2SO4 in grams which must be added to 500 ml of 0 2 M NH3 to yield a solution of pH 9 K for NH 2 10 5 1 3 248 g 3 1 320 g 2 4 248 g 4 6 248 g

Physical Chemistry
Surface chemistry14 Which statement is not true 1 pH of 1 x 108 M HCl is 8 2 96500 coulomb deposits 1 g equivalent of cop 3 Conjugate base of H PO4 is HPO 4 pH pOH 14 for all aqueous solution

Physical Chemistry
SolutionsLowering of vapour pressure for 1m aqueous solution is 1 08 mm of Hg at 25 C The vapour pressure of pure liquid at 25 C is 10x mm of Hg The value of x will be assuming very dilute solution 2 6 1 4 3 8 4 3

Physical Chemistry
Chemical kineticsQ 38 A first order reaction is 75 complete after 32 min when was 50 of the reaction completed O 16 min O 8 min 4 1 00 19 O 4 min

Physical Chemistry
Equilibrium0 The degree of dissociation of water at 25 C is 1 9 107 and density is 1 0 g cm The ionic constant for water is 1 1 0 x 10 10 3 1 0 10 16 2 1 0 10 4 4 1 0 10 8

Physical Chemistry
Solutionsd 0 1 form a pentamer The value of van t Hoff factor i A solute X when dissolved in a solvent associates to for the solute will be a 0 5 c 0 2 b 5 d 0 1 the freezing point of a 0 5 m KC

Physical Chemistry
Chemical Bonding1 The following equilibrium is established when HC1O4 is dissolved in weak acid HF HF HC104 CIO4 H F Which of the following is correct set of conjugate acid base pair 1 HF and HC104 3 HF and H F 2 HF and ClO 4 HC1O4 and H F
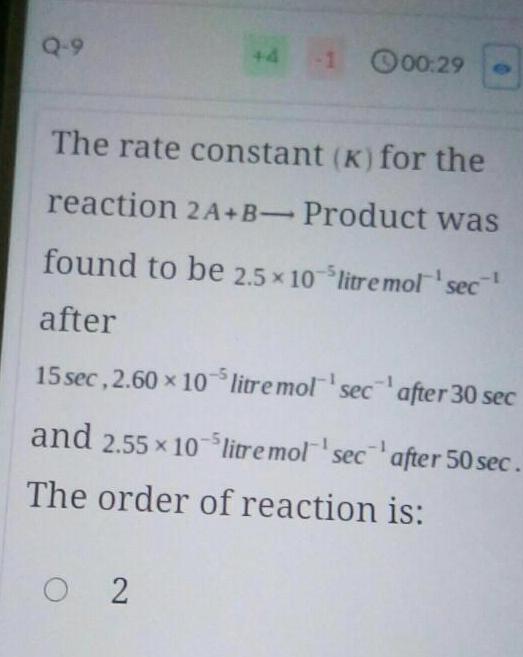
Physical Chemistry
Chemical kineticsQ 9 4 00 29 The rate constant K for the reaction 2A B Product was found to be 2 5 10 litre mol sec after 15 sec 2 60 x 105 litre mol sec 02 after 30 sec after 50 sec and 2 55 10 litre mol sec The order of reaction is

Physical Chemistry
Atomic Structure0 The energy of an electron in the first Bohr obit of H atom 50 13 6 e Chemical Arithmetic Atomic Structure is 13 6 eV Then which of the following statement s is are correct for He I The energy of electron in second Bohr orbit is 13 6 eV II The kinetic energy of electron in first orbit is 54 46 eV III The kinetic energy of electron in second orbit is 13 6 eV IV The speed of electron in the second orbit is 2 19 106 ms 1 I and II 2 I II and III 3 All are correct 4 None of these max 18 electron s are possible with nx conditio F1 1 facitu ar s 13 6 V 11 94 R H N 54 46 III fa IV 1 2 I II e III 3 4 13 6 el 2 1

Physical Chemistry
Equilibrium5 When glycinium hydrochloride NH CH COOH HCI is titrated against NaOH pH at the first half equivalence point is 2 40 and the pH at second half equivalence point is 9 60 The pH at first equivalence point is a 2 40 c 6 00 b 9 60 d 7 20

Physical Chemistry
GeneralQ 24 4 1 O 1 g 00 21 O Plot of log x m against log p is a straight line inclined at an angle of 45 When the pressure is 0 5 atm and Freundlich parameter k is 10 the amount of solute adsorbed per gram of adsorbent will be log 5 0 6990

Physical Chemistry
General68 Chromium plating can involve the electrolysis of an electrolyte of an acidified mixture of chromic acid and chromium sulphate If during electrolysis the article being plated increases in mass by 2 6 g and 0 6 dm of oxygen are evolved at an inert anode the oxidation state of chromium ions being discharged must be assuming Cr 52 and 1 mole of gas at room temperature and pressure occupies a volume of 24 dm a 1 c 1 b zero d 2

Physical Chemistry
General3 1 0 2 4 None of these 7 A graph between log t 2 and log a abscissa a being the initial concentration of A in the reaction for reaction A Product the rate law is log a 1 2 k 3 d A dt d A dt d A dt d A Slope 1 log a K K A Chemical k K A KIAN

Physical Chemistry
Energetics2 O For the reaction at 25 C X 04 1 2XO g AH 2 1 Kcal and AS 20 cal K The reaction would be 1 Spontaneous 2 Non spontaneous 3 At equilibrium 4 Unpredictable For the reaction 298 K 2A B C 70 4 n 3 1 1 m 1 s 25 C X 04 1 2XO g 31 3 AH 2 1 Kcal AS 20 cal K 1 Fad 2 3 3 4 71 298 KT 2A B C

Physical Chemistry
GeneralIn a sample of blood at 25 C H 4 6 x 108 M Find the molar concentration of OH and indicate whether the sample is acidic basic or neutral OH i M blood is A certain acid has a pKa equal to 4 88 Is this aci stronger or weaker than acetic acid which has a pKa of 4 74 What is the value of Ka for this acid It is a acid Ka Why do small highly charged hydrated metal ions form acidic solutions Small highly charged hydrated metal ions form acidic solutions because the water hydrates the metal ion then the high charge density of the metal ion turn hond this in the oxygen hydrogen

Physical Chemistry
Chemical kineticsO 00 19 m n 0 The rate law for a reaction between the substances A and B is given by rate k A B On doubling the concentration of A and halving the concentration of B the ratio of the new rate to the earlier rate of the reaction will be as

Physical Chemistry
GeneralQ 33 4 1 00 05 N g 3H g 2NH g 22 kcal The activation energy for the forward reaction is 50 kcal What is the activation energy for the backward reaction 72 kcal O 28 kcal 28 kcal

Physical Chemistry
Chemical kineticsQ 16 O 4 Find the two third life 2 of a first order reaction in which k 5 48 x 10 4 per second 8 201 10 s 2 01 10 S O 201 10 0 s 00 47 0 201 10 s O

Physical Chemistry
Electrochemistryc 0 91 96 The E values for Cr Mn Fe and Co are 0 41 M3 M2 los sirvi 1 57 0 77 and 1 97V respectively For which one of these metals the change in oxidation state from 2 to 3 mis is easiest a Co c Fe b Mn d Cr 1 00

Physical Chemistry
General4 a It minimum wavelength in emission spectrum of Li 2 in 51 94 HE paschen series is P then what is the maximum wave length in emission spectrum of He in balmer series 1 P 2 P 4 P 30 F 2 Haf txa 1 P

Physical Chemistry
GeneralQ 28 O Na SO4 Among the electrolytes Na2SO4 CaCl2 Al2 SO4 NH4Cl the most effective coagulation agent for Sb S sol is O CaCl O Al SO4 3 4 1 NU 00 18

Physical Chemistry
SolutionsAll on No 26 versible reaction A B 2 C the equilibrium concentrations of A B and C are 2 M 2 M and 4 M respectively then Kc for

Physical Chemistry
Chemical kineticsQ 15 1 00 19 The rate constant is numerically the same for three reactions of first second and third order respectively Which one is true for rate of three reaction r r r

Physical Chemistry
GeneralA gaseous compound of nitrogen and hydrogen contains 12 5 by mass of hydrogen The density of the compound relative to hydrogen is 16 The molecular formula of the compound is A B C NH N H NH3 NU

Physical Chemistry
ElectrochemistryThe standard electrode potential for the two electrode A A and B B are respectively 0 5 V and 0 75 V The emf of the given cell A A a 1 B a 1 B will be O 1 25 V 1 25 V 0 25 V 0 25 V

Physical Chemistry
SolutionsEquivalent conductivity BaCl2 H SO4 and HCl are x x2 and x3 Scm eq at infinite dilution If conductivity of saturated BaSO4 solution is x Scm then Ksp of BaSO is 2 3 4 500x x1 x3 2x3 10 x1 82 2x 2 5x10x x x 2x 0 25x of 1

Physical Chemistry
ElectrochemistryI 0 5 g of fuming H SO4 oleum is diluted with water This solution is completely neutralised by 26 7 mL of 0 4 N NaOH The percentage of free SO3 in the sample is 1 30 6 2 40 6 3 20 6 4 50 2 MnO 4HCl MnCl 2H O Ch the equivalent wt of HCI will be M Mol wt of HCl 1 M 2 M 2 3 2M 4 M 4 3 Number of moles of MnO 4 required to oxidise one mole of ferrous oxalate completely in acidic medium will be 1 0 6 mole 2 0 4 mole 3 7 5 moles 4 0 2 mole Bro Br 1 0652y Br 1 595 Then the species undergoing disproportionation is BrO 1 2 BrO 5 4 Consider the change in oxidation state of Bromine8 corresponding to different emf values as shown in the diagram below 1 82 V 1 5 V BrO Br 3 4 HBrO HBrO On the basis of the following E 0 values the strongest oxidizing agent is Fe CN Fe Fe e E 0 77 V 1 Fe CN 2 Fe 3 Fe 4 Fe CN 6 In the conversion H SO4 H S O8which process occurs Fe CN e E 0 35 V 1 Oxidation 2 Reduction 3 Oxidation as well as reduction 4 Neither oxidation nor reduction 7 In the reaction 2 Cut Cu Cu the equivalent weight of Cut is M is the mol wt of Cut 1 M M 2 4 1 3 4 2M Valency factor V for following redox reaction is respectively a As2 S AsO 0 b 1 1 10 c H3 PO PH3 H PO3 1 28 3 2 28 3 6 4 7 4 4 3 9 9 The oxidation number of phosphorus in Ba H PO 2 is 1 3 Page

Physical Chemistry
Solid stateA metal M having atomic mass 31 25 crystallizes in cubic close packing and it shows Schottky defects If the edge length of the cubic lattice is 500 pm and density of the metal is 1 6075 gm ml then calculate number of moles of metal atom M missing per litre of the crystal Given 1 amu 1 67 x 10 24 gm

Physical Chemistry
GeneralWhich of them is not a part of Dalton s atomic theory A B C D Atoms cannot be divided created or destroyed The number of protons in an atom is its atomic number In chemical reactions atoms are combined separated or rearranged All matter is composed of extremely small particles called atoms

Physical Chemistry
Chemical kinetics20 When a graph between log k and 1 T is drawn a straight line is obtained The points at which the line cuts y axis and x axis respectively correspond to the temperatures 1 0 E 2 303R logA 3 0 logA 2 E R en A 4 log A