Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Chemical BondingFor the reaction X s Y s Z g the plot of In PZ versus Pz pe T solid line where pz is the pressure in bar of the gas Z at temperature T and pe 1 bar Pz In po AH 3 7 Given d In K d R the gas constant R 8 314 J K mol 10 12 104 104 T K 1 is given below in where the equilibrium constant K and Pz po

Physical Chemistry
SolutionsColumn I CH3COCH3 CHCl3 A B C H5OH H O C C H5Br C H51 D CH3COCH3 CS Column II P ASmix 0 Q AV mix 0 R AHmix 0 S T Maximum boiling azeotrope Minimum boiling azeotrope

Physical Chemistry
Solutions100 ml of 1 M aqueous solution of acetic acid is mixed with 1000 ml of 0 1 M HNO at 25 C If 8 g of NaOH is added to the above solution The pH of the solution will be pK CH COOH 4 75 log 11 1 04 A 8 85 C 10 75 B 12 75 D 9 75

Physical Chemistry
Surface chemistryWhich one of the following is an incorrect statement for physisorption Question Type Single Correct Type N 1 It is reversible 2 It is multilayered English Review Q 3 It requires activation energy 4 It decreases with increasing temperature

Physical Chemistry
GeneralUpon heating with Cu S the reagent which do S not give copper metal is 1 CuFeS 2 CuO 3 Cu 0 4 CuSO4

Physical Chemistry
Gaseous and liquid states44 45 46 47 48 49 50 51 52 53 54 X 1 English Review Q Ideal gas has minimum density at 4 Question Type Single Correct Type 1 P 2 atm and T 200 K 2 P 2 atm and T 300 K 3 P 1 atm and T 250 K 4 P 2 atm and T 150 K

Physical Chemistry
GeneralQ 6 MCQ Marks 4 1 O If mass of a proton is 1 6 10 27 kg then number of moles of proton in 1 kg is 1 A 1 6 10 x6 022 10 3 B C 1 6x 10 27 6 022x102 6 022x1023 1 6x102

Physical Chemistry
Chemical BondingQuestion No 69 Single Correct A A Identify the number of compounds which can exist 3 PbF4 Bils TIF3 Tel3 2 3

Physical Chemistry
Generalsublimation energy Na S 650 kj mole Dissociation energy Cl 2 400 kj mole ionisation energy Na g 500kj mole Electron gain enthalpy Cl g 350kj mol e Using born haber cycle find the value o f lattice energy in KJ mole Please expl ain the entire process

Physical Chemistry
Chemical kineticsHydrogenation of vegetable ghee at 25 C reduces pressure of H from 2 atom to 1 2 atom in 50 minute The rate of reaction in terms of molarity per second is 1 1 09 x 10 6 2 1 09 x 10 5 3 1 09 x 10 7 4 1 09 x 10 8

Physical Chemistry
Chemical kinetics2 On doubling the initial concentrations of both A and B there is a change by a factor 8 in that rate of the reaction The rate of this reaction is given by 1 rate K A B 2 rate K A B 3 rate K A B 4 rate K A B

Physical Chemistry
EnergeticsIf enthalpy of neutralization of a weak monobasic acid HA with NaOH is 7 7 kcal eqv them ionization enthalpy of HA is Enthalpy of neutralization of HCI with NaOH is 13 7 kcal 2 17 eqv 1 21 4 kcal eqv 3 19 7 kcal eqv 2 6 kcal eqv 4 12 kcal eqv 8

Physical Chemistry
EnergeticsIn the reaction BrO 3 aq 5Br aq 6H 3Br 1 2H O 1 The rate of appearance of bromine Br is related to rate of disappearance of bromid ions as following 1 d Br dt 3 5 d Br dt 2 d Br dt 5 3 d Br dt 3 d Br dt 5 3 d Br dt 4 d Bral dt 3 5 d Br1 dt

Physical Chemistry
Surface chemistryA gaseous mixture of C H4 CH4 CO and N at 400 K 1 atm was added with excess of O subjected to sparking in a rigid container volume constant If pressure of CO obtained at 400 K in same volume is 0 9 atm and that of H O vapour is 0 8 atm If moles of C H and CH are equal then calculate the pressure of each gas in the original mixture 4 4

Physical Chemistry
ElectrochemistryOn passing a current through AgNO3 solution 5 4 g Ag is deposited at cathode assume 100 current efficiency If same current is passed through CuSO for same time only 0 8 g Cu is deposited then current efficiency in second experiment is 1 25 leon 2 50

Physical Chemistry
GeneralN For reaction aA L mM In condition of sudden volume increase degree of dissociation decreases it represents that Question Type Single Correct Type 1 a l m 2 a l m 3 a l m English Review Q 4 a l m 9

Physical Chemistry
Electrochemistry33 1 90 2 97 3 80 The Gibbs energy for the decomposition of Al2O3 2 3 4 3 3 at 500 C is as follow Al2O3 Al O 4G 966 kJ mol The potential difference needed for electrolytic reduction of Al2O3 at 500 C is at least AIEEE 2010 1 4 5 V 2 3 0 V 3 2 5 V 4 5 0 V The reduction potential of hydrogen half cell will

Physical Chemistry
EquilibriumCalculate the equilibrium Ni s 2Ag aq given that E Take 2 303RT F 1 1 10 7 3 6 1017 constant of the reaction 1 05 V cell Ni 2 2Ag s aq 0 06 2 1 1035 4 2 1020

Physical Chemistry
Solutionsanswer you will be awa When CaSO nH O is heated all of the water is driven off If 34 0 g of CaSO4 M mass 136 is formed from 43 0 g of CaSO4 nH O what is the value of n

Physical Chemistry
Chemical kineticsQ 7 Calculate the minimum amount of ethylene glycol molar mass 62g mol that must be added to 6 kg of water to prevent it from freezing at minus 0 3 c Given K for water 1 86K m

Physical Chemistry
GeneralIn order to balance the half reaction Cr O72 are added is Question Type Single Correct Type 15 2 9 English Review Cr the number of e H and H 0 2 7 14 6 3 6 14 7

Physical Chemistry
Chemical kineticsTYPE questions Fach question has 4 Consider the following statements a For zero order reaction t 2 is directly proportional to the initial concentration of the reactants b Inversion of cane sugar is an example of pseudo first order reaction c Decomposition of gaseous ammonia on a hot platinum surface is a zero order reaction at high pressure The correct statements are O a b only Read More Ob conly a c only

Physical Chemistry
SolutionsIn a 0 2 molar aqueous solution of a weak acid HX the degree of ionization is 0 3 The freezing point ikof the solution will be nearest to 1 0 48 C 3 0 36 C The I 2 0 48 C 4 0 26 C

Physical Chemistry
Gaseous and liquid states2 5 200 ml of O gas maintained at 700m pressure and 250 ml of N gas maintaine at 720mm pressure are put together in o litre flask If the temperature is kept co stant the final pressure of the mixture mm is 1 450 2 320 3 632 4 316

Physical Chemistry
GeneralConsider the following reaction in aqueous 54 solution 5 Br aq BrO aq 6H aq 3Br aq 3H O A If the rate of appearance of Br at a particular time during the reaction is 0 025 M sec what is the rate of disappearance in M sec of BrO at that time 1 0 025 M sec 3 0 075 M sec 2 0 042 M sec 4 0 0083 M sec Bro 0 025 M sec fra in M sec and fre facf Br f W 5 Br aq BrO aq 6H aq 3H O l 1 0 025 M sec 3 0 075 M sec at 3Br aq 2 0 042 M sec 4 0 0083 M sec

Physical Chemistry
GeneralA piece of parmesan cheese is burned in a soda can calorimeter and heats up 0 235 kg of water by 2 95 C The calorimeter was calibrated and had a calorimetry constant of 8 50 kcal kg C Given that protein contains 4 00 kcal g calculate the mass in g of the protein contained in the parmesan Assume that the cheese is composed primarily of protein

Physical Chemistry
Solid stateIn BeO Zinc Blende structure Mg is introduced in available tetrahedral voids Then ions are removed from a single body diagonal of the unit cell What will be the molecular formula of the unit cell A Be4Mg4O4 C Be3Mg303 B D Be Mg4O2 Be3Mg303 75

Physical Chemistry
Chemical BondingBoiling point of H Se is higher than H S this is 82 best explained by 1 Highest extent of hydrogen bonding in H Se 2 Higher polarity of H S 3 Higher polarity of H Se 4 Higher dispersion forces in H Se due to its higher molecular weight

Physical Chemistry
Chemical kinetics1 Following figure shows a graph in log 0K vs T where K is rate constant and T is temperature and an The straight line BC has slope intercept of 5 on Y axis Thus Ea the energy of activation is log K B 1 2 303 x 2 cal 1 2 303 1 T C 2 2 2 303 cal

Physical Chemistry
Atomic StructureThe wavelengths of the Ka X ray radiations from two sources P and Q are 1 50 ar 75 A 1 A 10 10m Then most likely A P 41Nb Q 29 Cu C P 29Cu Q 41 Nb B P 29Cu Q 47 Ag D P 41Nb Q 27 Co

Physical Chemistry
Electrochemistry2 A 20 0 mL sample of Nalo was treated with an excess of KI and HCl and the iodine produced titrated with 0 05125 M Na S O If 19 35 mL of Na S O solution were required to reach a starch iodine endpoint what was the concentration of the NalO
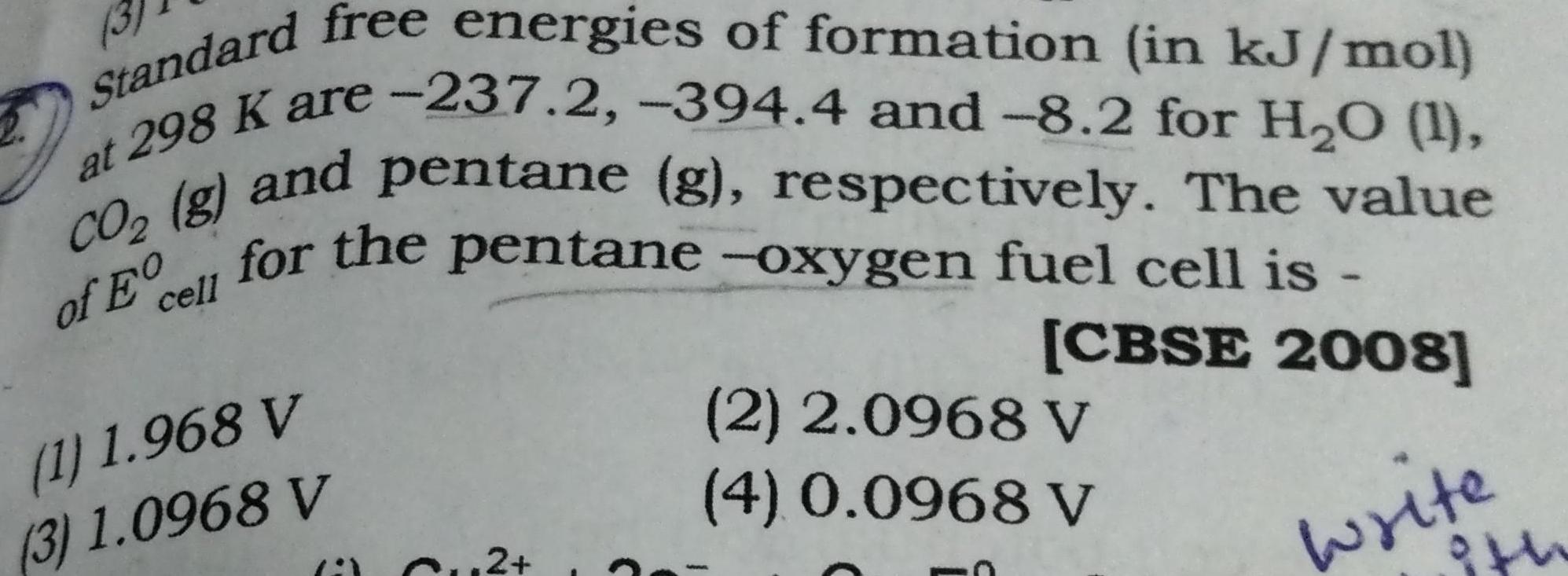
Physical Chemistry
EnergeticsStandard free energies of formation in kJ mol at 298 K are 237 2 394 4 and 8 2 for H O 1 of Ecell for the pentane oxygen fuel cell is CO g and pentane g respectively The value CBSE 2008 write 1 1 968 V 3 1 0968 V Cu2 2 2 0968 V 4 0 0968 V

Physical Chemistry
Atomic StructureALLEN 0 1 mole of each of A B and A B compounds 69 have mass of 4g and 8g respectively What are the atomic masses of A and B respectively in amu 1 20 10 2 40 30 3 30 40 4 10 20 A B A B 4g 8g 0 1 AB am 1 20 10 2 40 30 3 30 40 4 10 20 AM

Physical Chemistry
Equilibrium9 At 527 C temperature the value of Ke is 4 for the reaction NH3 g what will be the value of Kp for reaction N2 g 3H2 g 2NH3 g 3 16 800 R X SOOR 2 2 g H2 g then 2 800 R 4 2 4 None of these

Physical Chemistry
GeneralA bulb of unknown volume V ideal gas at 2 atm pressure It was connecte to another evacuated bulb of volume 0 51 tre through a stopcock When the stopco was opened the pressure in each bulb be came 0 5atm Then V is 1 17 ml 2 1 7 L 3 0 17L 4 0 34

Physical Chemistry
EquilibriumIn 2 lit vessel following equilibrium is developed CO2 s CO2 g If equilibrium mixture is transferred to 4 lit vessel then find out partial pressure of CO2 g in atm after attaining to equilirbium assume presence of excess of CO2 s Orrect Answer 4 atm 4

Physical Chemistry
General2 Consider the following reaction sequence CaCl aq CO g H O CaCO3 s 2HCl aq heat CaCO3 s CaO s H O g If the percentage yield of the 1st step is 80 and that of the 2nd step is 75 then what is the expected overall percentage yield for producing CaO from CaCl 1 50 3 55 2 70 4 60

Physical Chemistry
Equilibriumhe equilibrium constant Ke is calculated using holar concentrations For gaseous reactions nother form of the equilibrium constant Kp is alculated from partial pressures instead of oncentrations These two equilibrium constants re related by the equation Kp K RT An here R 0 08206 L atm K mol T is he absolute temperature and An is the change in me number of moles of gas sum moles products um moles reactants For example consider the eaction N g 3H2 g 2NH3 g or which An 2 1 3 2 Part A For the reaction 2A g 3B g C g K 35 8 at a temperature of 313 C Calculate the value of Kp Express your answer numerically View Available Hint s Die Tau VO

Physical Chemistry
General24 An organic compound contains carbon hydrogen and oxygen Its elemental analysis gave C 38 71 and H 9 67 The empirical formula of the compound would be AIPMT Prelims 2008 1 CH O 3 CH Q 2 CH O A CHO

Physical Chemistry
Generald Ca ions are impo 49 The product obtained as a result of a reaction of nitrogen with CaC is a Ca CN c CaCN b Ca CN 2 d CaCN 3 11 1 NEET Phase 1 2016 WB JEE 2017

Physical Chemistry
Atomic Structure9 a Elements A B C and D have atomic numbers 12 19 29 and 36 respectively On the basis of electronic configuration write to which group of the periodic table each element belongs b Predict the blocks to which these elements can be classified Also predict their periods and groups c Which of these are representative elements

Physical Chemistry
Solutions27 Which is an incorrect statement O a Sea water is converted into fresh water based on reverse osmosis b The best colligative property to determine molecular mass of protein is osmotic O pressure O c The phenomenon of osmosis was first observed by Faraday d Silica gardens are formed on the basis of osmosis

Physical Chemistry
Equilibrium3 21 44 59 AB g is dissociates as 4 5 36 1 AB g AB g B g when the initial pressure of AB is 800 torr and the total pressure developed at equilibrium is 900 torr What percentage of AB g is dissociated 1 10 2 20 3 25 4 30 89 3 21 44 AB g 3 AB g AB g B g aptar facifar dr AB80A TA TE AB faci fara 900 1 10 4 5 36 2 20 3 25 4 30

Physical Chemistry
Chemical kineticsFrom the following data for the reaction between A and B A B Initial rate at mol Lit sec mol Lit 2 5 10 5 0 10 1 0 10 mol Lit 3 0 10 5 6 0 10 6 0x10 5 300 K Overall order of this reaction is 3 5 0 10 4 0 10 1 6 10 2 Now select the correct statement s of the following 320 K 2 0 10 Units of rate constant of the above reaction is mol Lit sec Rate constant of this reaction at 320 K is 2 66 108 mol Lit sec Rate constant of this reaction at 320 K is 12 66x107 mol Lit sec Clear Re

Physical Chemistry
Electrochemistrywhen mixed in solution copper and ammonia form the complex ion Cu NH3 4 2 The formation constant is 1 70 10 13 If a solution initially contains 0300M Cu NO3 2 and 300M NH3 what is the molarity of Cu2 at equilibrium

Physical Chemistry
Solid stateA compound X Y form crystal in which anion Y form hexagonal closes packing and cation occupy only two third of octahedral hales Its P E P is A 74 9 B 74 C 77 6 D 72 A compound XY O form crystal in which oxides form cubic closest backing X ion occupy in one eighteen of tetrahedral voids and Y3 ions occupy one half of octahedral voids Hence P E P of crystal

Physical Chemistry
Chemical kineticsA gaseous reaction A g B g C g shows increase in pressure from 100 mm te 120 mm in 5 minutes The rate of disappearance of A is 1 4 mm min 1 3 16 mm min 1 2 8 mm min 1 4 2 mm min 1

Physical Chemistry
SolutionsCarbonated beverages are prepared by pressurizing carbon dioxide into the solution A fraction of carbon dioxide dissolves in the beverage and the remainder stays in the gas phase between the surface of the liquid and the lid at pressures ranging from 2 00 bar to 8 00 bar Calculate the range of molarities of carbon dioxide in a beverage Ans c CO from 0 0678 mol L to 0 711 mol L

Physical Chemistry
GeneralA sulphide ore first converted into its oxide before reduction This is done because 1 A sulphide ore cannot be reduced to metal at all 2 No reducing agent is found suitable for reducing a sulphide ore 3 The enthalpy of formation of CO is more than that of CS 4 A metal oxide is generally less stable than the metal sulphide 74 44 Rafa fafan 1 is 2 314 1 a safe for a 14 3 CO af CS fat ant tr ci fe 4 STERIS 414 4041 A CHISS

Physical Chemistry
Energetics14 The lattice enthalpy of KI will be if the enthalpy 1 AH KI 78 0 kcal mol 1 II lonisation energy of K to K is 4 0 eV III Dissociation energy of l to 1 is 28 0 kcal mol IV Sublimation energy of K is 20 0 kcal mol V Electron gain enthalpy for I to 1 is 70 0 kcal mol 1 VI Sublimation energy of 12 is 14 0 kcal mol 1 1 eV 23 0 kcal mol 1 14 1 kcal mol 2 14 1 kcal mol 3 141 kcal mol 1 A