Chemical Bonding Questions and Answers

Physical Chemistry
Chemical Bonding1 dox NEET VOL 11 In the reaction K O KO 1 O acts as an oxidising agent 2 Both K and O are oxidised 2 3 O is oxidised with K is reduced 4 K acts as an oxidising agent 12 Cr O X Cr H O oxidised product of X X in the above reaction cannot be 2 1 C O 2 Fe 3 SO 4 S

Physical Chemistry
Chemical BondingThe IUPAC name of the compound ur is 1 3 Keto 2 methylhex 4 enal 3 5 Methyl 4 oxohex 2 en 5 al Statement 1 For adsorption AG AH AS all have negative values 2 5 Formylhex 2 en 3 one 4 3 Keto 2 methylhex 5 enal

Physical Chemistry
Chemical BondingChoose the incorrect option regarding following complex is K Pt Cl cl x O z x Bond length Odsp hybridisation O O S of Pt 2 O C C bond length in this complex is less than individual CH CH CH CH M

Physical Chemistry
Chemical BondingWhat is the dominant intermolecular force or bon that must be overcome in converting liquid CH O to a gas 1 London dispersion force 2 Hydrogen bonding 3 Dipole dipole interaction 4 Convalent bonds

Physical Chemistry
Chemical BondingA B 3 and the percentage error in the measurements of A B C and D are 4 2 3 and 1 respectively CD3 2 Example 12 Find the relative error in Z if Z


Physical Chemistry
Chemical BondingChoose incorrect statement regarding alkaline earth metal compounds Solubility of hydroxides increases down the group Solubility of carbonates decreases down the group Tendency of nitrates to form hydrates increases down the group Tendency of halides to form hydrates decreases down the group

Physical Chemistry
Chemical BondingThe dissociation constants for acetic acid and HCN at 25 C are 1 5 x 10 5 and 4 5 x 10 10 respectively The equilibrium constant for the equilibrium CN CH3COOHHCN CH3COO would be A 3 0 x 105 B 3 0 X 10 5

Physical Chemistry
Chemical BondingALLEN is a un CAREER INSTITUTE KOTA RAJASTHAN IV XeO 4 D I II III In which of the following species presence of double bond does NOT affect idealised bond angle re 1 POCI III XeO F C III IV only II XeO F B I II only fa

Physical Chemistry
Chemical BondingA standard hydrogen electrode has zero electrode potential because 1 Hydrogen is easiest to oxidize 2 This electrode potential is assumed to be zero 3 Hydrogen atom has only one electron 4 Hydrogen is the lightest element

Physical Chemistry
Chemical Bonding3 a chloroacetaldehyde is more reactive than acetaldehyde because a chlorine atom has I effect and decreases ve character of carbony carbon b chirioncatom has I effect and decreases ve charactor of carbony carbon c Methyl group has 1 effect and increases ve charactor of carbony carbon d chiorine atom has I effect and increses ve character of crbonyl carbon

Physical Chemistry
Chemical Bonding49 For the synthesis of ammonia from its elements 3H g N g 2NH g the equilibrium constant K 6x 10 at 300 K and AH 24 kCal mol Calculate equilibrium constant at 600K assuming no change in AH between 300 K and 600K Given e 20 2 10 isted in the table are forward and reverse rate constants for the reaction 2NO g N g 0 g k M s k M s 1 1 x 10 6

Physical Chemistry
Chemical BondingC Change of medium from acidic to D Change in medium from acidic to basic will change the nature of product A sample of steel weighing 0 30 g was subjected to a chemical reaction to convert its sulphur impurity to H S g The evolved gas required 2 40 mL 0 02 N iodine solution Which of the following statement s is are true A B C A lodine is reduced to iodide B H S is oxidized to S C Steel contain 0 256 of S by weight D The reaction of H S with I is a precipitation reaction rather than a redox reaction TCH THE COLUMN

Physical Chemistry
Chemical Bonding8 What is the mole fraction of the solute in a 1 00 m aqueous solution CBSE AIPMT 2015 a 0 177 b 1 770 c 0 0354 d 0 0177

Physical Chemistry
Chemical Bondingelectrons In which of the following pairs the radii of second species is greater than that of first 1 K Ca 2 H He 3 Mg Mg2 4 0 0

Physical Chemistry
Chemical Bondinga 95 c 75 d 65 Al2 SO4 3 solution of 1 molal concentration is present in 1 litre solution of density 2 684 s cc How many moles of BaSO 4 would be precipitated on adding excess of BaCl2 in it b 3 moles a 2 moles c 6 moles 5 A certain public water supply contains 0 10 ppb part per billion of chloroform CHCI 3 How d 12 moles selecules of CHCl would be obtained in 0 478 ml drop of this water

Physical Chemistry
Chemical BondingOxalic acid H C O4 reacts with permanganate ion according to the balanced equation below How many mL 0 0154 KMnO4 solution to react 25 0 mL of 0 0208 M H C 04 solution 5H C O4 aq 2MnO4 6H aq a 13 5 mL c 33 8 mL 2Mn aq 10CO g 8H O 1 b 18 5 mL d 84 4 mL

Physical Chemistry
Chemical Bonding1 An alkene obtained by the dehydration of an alcohol 4 on ozonolysis gives molecules of acetaldehyde for every molecule of alkene The alcohol 4 is a CH3CH CH OH c CH3CH CHCH OH b CH3CH OH d CH3CH CHCH3

Physical Chemistry
Chemical BondingThe elements whose atoms have three outer most shells incomplete are called 1 s block elements 2 p block elements 3 f block elements 4 d block elements

Physical Chemistry
Chemical BondingWhich pair of elements has the same characteristic chemical properties 1 Z 13 Z 22 2 Z 3 Z 11 3 Z 4 Z 24 Z 2 Z 4 4 4

Physical Chemistry
Chemical BondingThree moles of an ideal gas are taken through a cyclic process ABCA as shown on T V diagram in the figure The gas loses 2510 J of heat in the complete cycle If TA 100 K and TB 200 K The work done by the gas during the process BC is R 8 3 J mol K Tel Te TA 1 VA Vc O 5000 J O 5000J O 4000 J VB

Physical Chemistry
Chemical BondingGeneral molecular formula of carbonyl compounds is 1 C H O 3 C H O 2 C H2n 2O 4 C H2n 2O

Physical Chemistry
Chemical BondingThe oxidation potential of Zn Cu Ag H and Ni are 0 76 0 34 0 80 0 0 55 volt respectively Which of the following reaction will provide maximum voltage 1 Zn Cu Cu Zn 2 Zn 2Ag 3 H Cu 4 H Ni 2 2Ag Zn 2H Cu 2H Ni

Physical Chemistry
Chemical Bonding51 Which one of the following is expected to exhibit optical isomerism en ethylenediamine a cis Pt NH3 2Cl b trans Pt NH3 2Cl Le cis Co en Cl d trans Co en Cl 2005
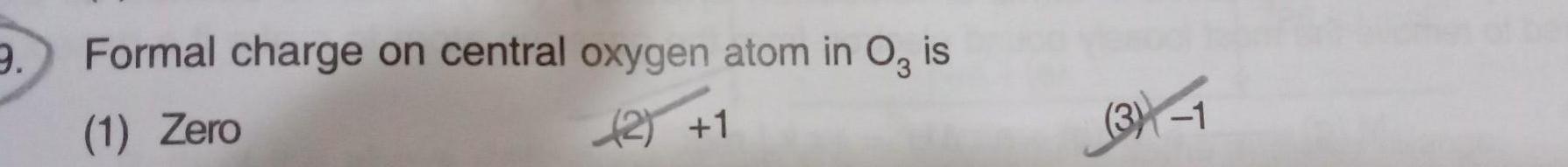

Physical Chemistry
Chemical Bonding1 Which complex compound will give four isomers a Fe en 3 Cl3 b Co en Cl Cl c Fe PPh3 3NH ClBr Cl d Co PPh3 3Cl Cl3 2000

Physical Chemistry
Chemical BondingAt 300 K and 1 atm 15 mL of a gaseous hydrocarbon requires 375 mL air containing 20 O by volume for complete combustion After combustion the gases occupy 330 mL Assuming that the water formed is in liquid form and the volumes were measured at the same temperature and pressure the formula of the hydrocarbon is Main 2016 Offline 4 C H10 1 C H 2 C H JEE 3 C Hg

Physical Chemistry
Chemical Bondingch Q 36 A solid PQ has rock salt type st toms are at the corners of the unit cell If the body centred toms in all the unit cells are missing The resulting 11

Physical Chemistry
Chemical Bondingacceleration of electron in 1st orbit of He is acceleration of electron 2rd orbit of Be is B 20 C 1 5 D i 4

Physical Chemistry
Chemical BondingIf force of attraction between the electron and nucleus in 2nd orbit of Li isf force of attraction if electron present in 1 orbit of His A f B 12 f C f 81 D 16 f 27

Physical Chemistry
Chemical BondingWhich among the following will be named as dibromidobis ethylene diamine chromium III bromide 1 Cr en Br Br 3 Cr en Br Br 2 Cr en Br 4 Cr en Bra

Physical Chemistry
Chemical BondingWhich of the following will give maximum number of isomers 1 Copy NH g 3 3 Fe C O en 1 2 Nien NH 4 Cr NO NH

Physical Chemistry
Chemical BondingThe effective atomic number of Cr atomic no 24 in Cr NH 1 35 Cl is 2 27 3 3 33 4 36

Physical Chemistry
Chemical Bonding5 How many unpaired electrons are present in the Brown Ring complex Fe H O NO SO4 1 4 3 0 2 3 4 5

Physical Chemistry
Chemical BondingThe number and type of bonds between tw boron atoms in B molecule 1 Two sigma two pi 2 Two sigma one pi 3 One Pi

Physical Chemistry
Chemical BondingAmongest the following highest paramagnetism 1 Cr H O 1 3 Cu H O 1 ions which one has the 2 Fe H O 4 Zn H O

Physical Chemistry
Chemical BondingWhich of the following is considered to be an anti cancer species H N X Pf CI 1 3 CL CI Pt CI NH CI CI 2 CI 4 P CH TI CH CI H N X Pt H N

Physical Chemistry
Chemical BondingWhich gives only 25 mole of AgCl when reacts with AgNO3 1 PtCl 4NH 3 PtCl 4NH3 2 PtCl 5NH3 4 PtCl 3NH 4

Physical Chemistry
Chemical BondingThe EAN of cobalt in the complex ion Co en Cl is 1 27 2 36 3 33 4 35

Physical Chemistry
Chemical BondingThe two compounds sulphato penta ammine cobil III bromide and sulphato penta ammine cobali III chloride represent 1 Linkage isomerism 2 Ionisation isomerism 3 Co ordination isomerism 4 No isomerism

Physical Chemistry
Chemical BondingThe charge present on 42 g of nitride ions coulomb is 1 4 8 10 19 3 2 86 105 2 14 4 10 19 4 8 6 105

Physical Chemistry
Chemical Bonding4 In an octahedral structure the pair of d orbitals involved in sp of hybridisation is 1 d x y dz 2 dxz d2 3 d dxz 4 dxy dyz smpia sot to600 Samole

Physical Chemistry
Chemical BondingWhich of the following electron transitions in a hydrogen atom will require the largest amount of energy 1 from n 1 to n 2 2 from n 2 to n 4 3 from n 5 to n 1 4 from n 3 to n 5

Physical Chemistry
Chemical BondingThe values of electronegativity of atoms A and B are 1 2 and 4 0 respectively The ionic character of the A B bond is DPMT 2009 b 72 24 d 4 3 a 50 c 55 3

Physical Chemistry
Chemical BondingArrange the following molecules in the increasing order of their ionic character 2 Points LiF K O N SO2 Cu LiF K 0 N SO CIF3 N K 0 LiF SO CIF3 N K 0 LiF CIF3 SO

Physical Chemistry
Chemical Bondingf we have 10 molal urea solution Calculate mole fraction of urea in this solution also calculate o w w of urea MW 60 O moles urea in 1000 g of water


Physical Chemistry
Chemical Bondingd Many important compounds in the chemical industry are derivatives of ethylene C2H4 One is methyl methacrylate What are the indicated bond angles in methy methacrylate angle d angle e angle f H Methyl methacrylate 90 109 50 120 1800

Physical Chemistry
Chemical Bonding3 C O F CI NO CN 1 The overall formation constant of the Co NH 2 ion in aqueous solution is 105 and the standa potentials for the reduction of Co aq and Co NH 1 aq are as follows Co aq eCo aq E 1 9V Co NH3 aq e Co NH3 aq E 0 1V Calculate the nearest overall formation constant of the Co NH3 ion www 1 1030 2 1033 3 1035 4 1038 iment corresponding

Physical Chemistry
Chemical BondingWe know the reaction between Zinc and sul phuric acid and as a product it releases the H2 gas Now if we take some amount of so dium hydroxide in place of sulphuric acid an d react it with zinc what will be the change i n amount of hydrogen gas produced a Same amount of H2 gas is evolved b H2 gas is not evolved c The amount of H2 gas evolved is much I ess d In place of H2 gas 02 gas is evolved