Chemical kinetics Questions and Answers

Physical Chemistry
Chemical kinetics3 The entropy of vaporization of benzene is 85 JK mol When 117g benzene vaporizes at it s normal boiling point the entropy change of surrounding is 1 85 JK 2 85 x 1 5 JK 3 85 1 5 JK 4 None of these
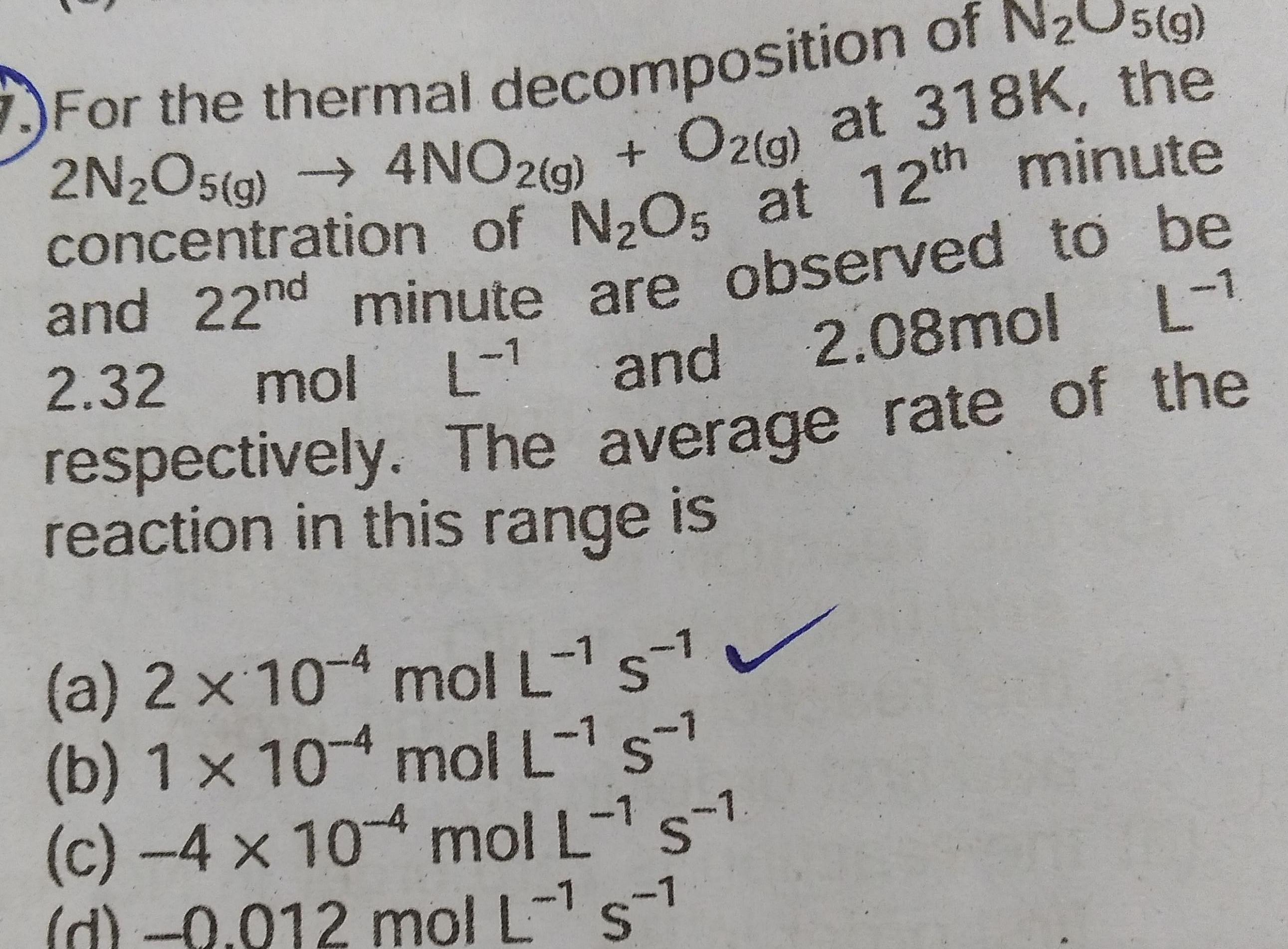
Physical Chemistry
Chemical kineticsFor the thermal decomposition of N O5 g 2N2O5 g 4NO2 g O2 g at 318K the concentration of N O5 at 12th minute and 22nd minute are observed to be 2 32 mol L and 2 08mol L respectively The average rate of the reaction in this range is a 2 x 10 4 mol L 51 b 1 x 104 mol L s c 4 x 10 4 mol L s 1 d 0 012 mol L s 1 1

Physical Chemistry
Chemical kineticsExcluded volume of a gas will be larger if 2 Large 4 less than 1 1 Small 3 1 is

Physical Chemistry
Chemical kinetics2 Which of the following is are correct for the first order reaction log a x T112 Time I Initial Conc a III 1 I log a a x rate 2 11 Time 4 M II Initial Conc a IV

Physical Chemistry
Chemical kinetics4 The conversion of A to B follows a second order kinetics Doubling the concentration of A will increase the rate of formation of B by a factor of c 4 a 1 2 b 2 ANGESPULEATPASION IN d 4

Physical Chemistry
Chemical kineticsWhat is the relationship between coefficients of reactants in a balanced equation for an overall reaction and exponents in rate law In what case the coefficients are the exponents

Physical Chemistry
Chemical kineticsTwo first order reactions proceed at 25 C at the same rate The temperature co efficient of the rate of first reaction is 2 and that of the second reaction is 3 Find the ratio of rates of the reaction second and first reaction at 75 C 1 7 6 3 10 4 2 9 5 4 12 6

Physical Chemistry
Chemical kineticsILLUSTRATION 11 Show that in a first order reaction time required for completion of 99 9 is 10 times of half life 2 NCERT of the reaction

Physical Chemistry
Chemical kineticsThe specific reaction rate of a first order reaction is 9 212 x 10 2 s If the reaction started with 2 mol L 1 calculate the amount of the reactant remaining after 50 seconds a 1 36 mol L 1 c 0 22 mol L b 0 632 mol L 1 d 0 02 mol L

Physical Chemistry
Chemical kineticsThe following graph shows how t 2 half life of a reactant R changes with the initial reactant concentration ao 1 2 1 a The order of the reaction will be 1 O 3 2 2 1 4 3

Physical Chemistry
Chemical kineticsThe temperature coefficient for the saponification of ethyl acetate by NaOH is 1 75 The activation energy is log 1 75 0 243 1 10 2 kcal mol 1 3 30 kcal mol 1 2 15 4 kcal mol 1 4 40 kcal mol 1

Physical Chemistry
Chemical kineticsFor a chemical reaction aA bB cC dD The ratic of rate of disappearance of A to that of appearance of C is 1 0 0 2 C a

Physical Chemistry
Chemical kineticsILLUSTRATION 4 Identify the reaction order from the following rate constants NCERT 1 k 2 3 x 10 5 L mol 1 s 1 CA

Physical Chemistry
Chemical kineticsIf the rate of reaction is 2 6 10 3 mol L 1s 1 at 50 C and 7 02 10 2 mol L 1s 1 at 80 C then what will be the temperature coefficient of the reaction

Physical Chemistry
Chemical kineticsILLUSTRATION 9 A first order reaction is found to have a rate constant k 5 5 x 10 14 s 1 Find the half life of the reaction NCERT

Physical Chemistry
Chemical kineticsFor a reversible reaction of the type mAnB it was found that the concentration of A and B are the same at equilibrium k and k are the rate constants of the forward and backward reaction at a given temperature Which of the following relations is correct

Physical Chemistry
Chemical kineticsA three step reaction takes place such that net rate k k 3 k constant is k Ae 9 RT K Ae 10 RT K3 Ae 7 RT

Physical Chemistry
Chemical kineticsThe activation energy for the reaction 2HI H 1 is 184 kJ mol How many times greater is the rate constant for this reaction at 520 C than at 500 C R 8 31 JK 1 mol 1 1 0 5 3 5 5 2 0 18 4 2

Physical Chemistry
Chemical kineticsQ7 The following data are for the decomposition of ammonium nitrite in aqueous solution Volume of N in c c 6 25 9 0 11 40 Time minutes 10 15 20 The order of reaction is 1 zero 3 two 13 65 35 05 25 2 one 4 three

Physical Chemistry
Chemical kineticsFor a gaseous phase reaction 2A B 2AB the following rate data was obtained at 300K Rate of disappearance Concentration of B mol litre min i 1 8 10 3 ii 1 08 10 iii 5 4 10 3 The rate constant for the reaction is 1 0 5 mol 1 min 1 litre 2 0 8 mol min litre A 0 015 0 09 0 015 B 0 15 0 15 0 45

Physical Chemistry
Chemical kineticsThe decomposition of ozone proceeds as 1 03 0 0 fast ii 0 03 202 slow The rate expression should be 1 Rate K 031 2 2 3 Rate K 03 0 Rate K 03 0 022016 4 Rate K 03 0 1 1

Physical Chemistry
Chemical kineticsThe rate of a certain reaction depends on concentration according to the equation K C 1 K C dC dt at very very high concentration what will be the order of the reaction
Physical Chemistry
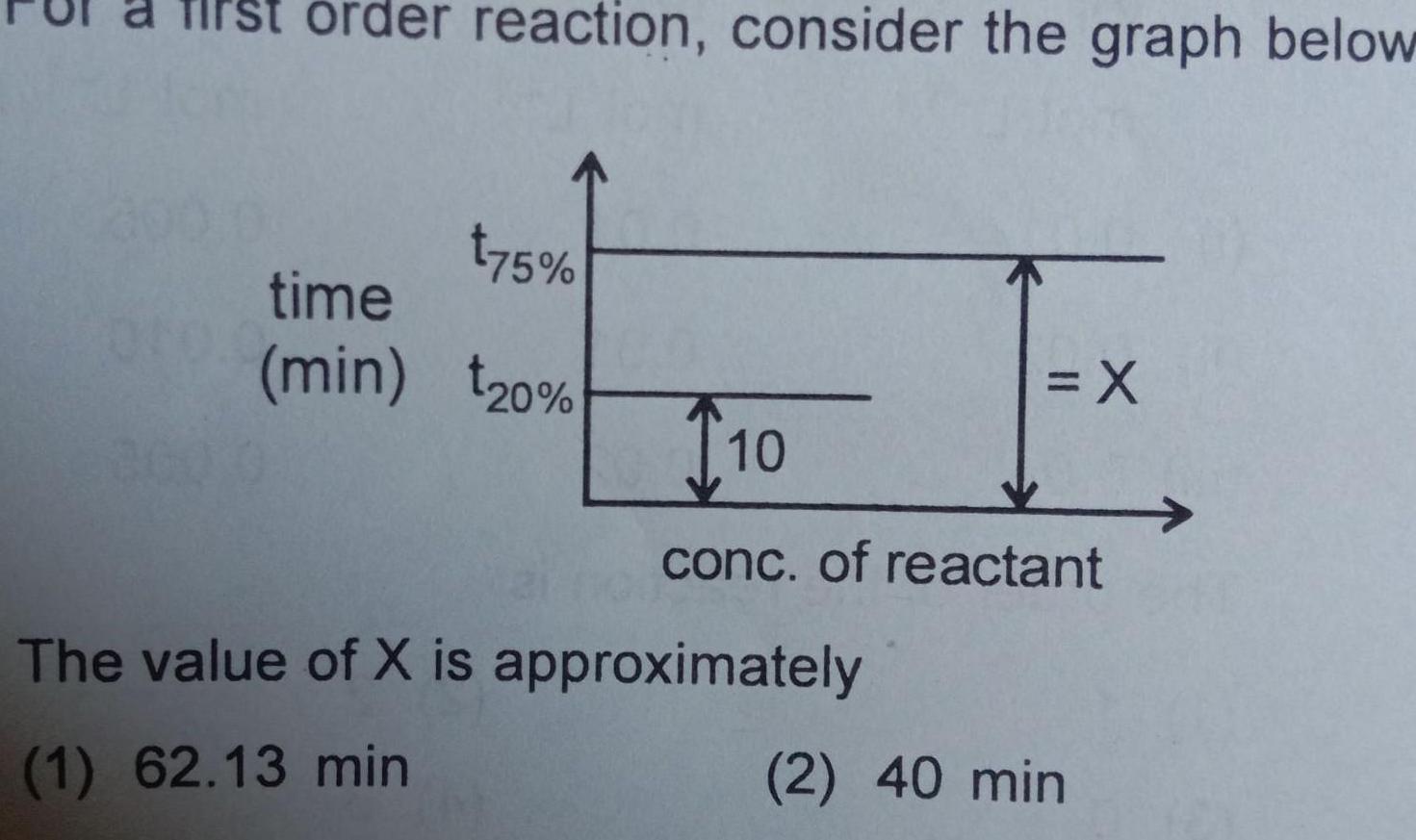
Chemical kineticsorder reaction consider the graph below t75 time min 20 X T10 conc of reactant The value of X is approximately 1 62 13 min 2 40 min

Physical Chemistry
Chemical kineticsVILLUSTRATION 16 The rate of the chemical reaction doubles for an increase of 10K in absolute temperature from 298K Calculate E

Physical Chemistry
Chemical kineticsFor the reaction 2H O 2H O O the rate equation is given as rate k H O T 1 alkaline medium The unit of rate for this reaction is 2 a mol L S c mol L S 1 b mol L s 1 d mol L s 1

Physical Chemistry
Chemical kineticsALLUSTRATION 10 Sucrose decomposes in acid solution into glucose and fructose according to the first order rate law with t 2 3 00 hours What fraction of the sample of sucrose remains after 8 hours

Physical Chemistry
Chemical kineticsx24 is a radioactive substance and it decomposes by a decay into stable pb306 The half life of decay of X into Pb is 138 6 days If 1 mole of X is placed in a closed tube then in 69 3 days the amount of a particle in moles accumulated in the tube is approx O 1 mole O 06 moles O 2 moles O 24 moles

Physical Chemistry
Chemical kineticsFor the decomposition N O N O 1 2 O the initial pressure of N O5 and after 20 s the pressure of reaction mixture becomes 133 mm Calculate the rate of reaction in terms of a change in atm s and b change in molarity s Given that reaction is carried out at 127 C is 114 mm 114 N O N O 1 2 02 2 g

Physical Chemistry
Chemical kinetics20 The following graph represents the total energy of homonuclear diatomic systems AA BB CC and DD as a function of their internuclear distance E O FB Based on the above graph select the correct statement A The molecule A is stable B The correct order of bond length is B D C C has a greater bond dissociation energy than D D A has a greater bond dissociation energy than B Page 7

Physical Chemistry
Chemical kineticsdP 11 The reaction 2NO g H g N O H O follows the rate law N O K PNG PH dt If the reaction is initiated with PNo 1000 mm Hg and PH 10 mm Hg then the reaction will foll NO 1 Third order kinetics 2 Second order kinetics 3 First order kinetics 4 Zero order kinetics

Physical Chemistry
Chemical kinetics12 Which of the following statements is incorrect a A second order reaction must be a bimolecular elementary reaction b A bimolecular elementary reaction must be a second order reaction c Zero order reaction must be a complex reaction d First order reaction may be complex or elementary reaction

Physical Chemistry
Chemical kineticsA radioactive element gets spilled over the floor of a room Its half life period is 30 days If the initial activity is ten times the permissible value after how many days will it be safe to enter the room 1 1000 days 3 10 days 2 300 days 4 100 days MAINS 2007

Physical Chemistry
Chemical kinetics3 In a reaction between A and B the initial rate of reaction To was measured for different initial concentrations of A and B as given below A mol L 1 0 20 B mol L 1 0 30 ro mol 0 20 0 40 0 10 0 05 L s 5 07 x 105 5 07 105 1 43 x 104 The order of the reaction with respect to A is a 1 5 b 0 5 c 1 d 2

Physical Chemistry
Chemical kinetics45 The activation energy for the reaction H O2 H O 0 1 2 is 18 K cal mol at 300 K calculate the fraction of molecules of reactonts having energy equa to or greater than threshold energy Anti log 13 02 9 36 x 10 14 1 9 36 x 10 14 2 1 2 x 10 12 3 4 2 x 10 16 4 5 2 x 10 15

Physical Chemistry
Chemical kineticsIn the reaction P QR S The time taken for 75 reaction of P is twice the time taken for 50 reaction of P The concentration of Q varies with reaction time as shown in the figure The overall order of the reaction is A 2 C 0 B 3 D 1 Qlo Q Time 201

Physical Chemistry
Chemical kinetics31 4 grams of NaOH are transferred to 100mL of 1M H SO solution Calculate the pH of Ans zero 4 the final mixture

Physical Chemistry
Chemical kinetics2 AH of water is 285 8 kJ mol If enthalpy of neutralization of strong monoacid with strong mono 57 3 kJ mol AH of OH ion will be 228 5 kJ mol 1 3 114 25 kJ mol 1 2 228 5 kJ mol 1 4 114 25 kJ mol 1

Physical Chemistry
Chemical kineticsreaction Initial moles are If a is the degree of dissociation and P is the total pressure then the partial pressure of PC13 is 1 2 3 4 b aa a b c ac a ad a b c ad a 1 a b c aa a b c aa b aa PC1 PC13 Cl a b C P atm P atm P atm P atm

Physical Chemistry
Chemical kineticsSOLUBILITY 27 Henry s law constant for dissolution of CH4 in benzene at 298 K is 2 x 105 mm of Hg Then solubility of CH4 in benzene at 298 K under 760 mm of Hg is 1 1 2 x 10 5 3 4 x 10 7 2 3 8 x 10 3 4 1 x 10

Physical Chemistry
Chemical kineticsWhich of the following rate law has an overall ord of 0 5 for reaction involving substances x y and z AIIMS 9 1 Rate K C C C X 2 Rate K C 0 5 C0 5 C 0 5 3 Rate K C 5 C C 4 Bata KICHC 161 2

Physical Chemistry
Chemical kinetics100 ml of liquid A and 25 ml of liquid B is mixed to give a solution which does not obey Raoult s law T volume of the solution 1 Will be 125 ml 3 Can be or than 125 ml 2 Can be or than 125 ml 4 Will be less than 125 ml pon 672 Kond pure nitric acid boils at 350 K TH

Physical Chemistry
Chemical kineticsPure water freezes at 273 K and 1 bar The addition of 34 5 g of ethanol to 500 g of water changes the freezing point of the solution Use the freezing point depression constant of water as 2 K kg mol The figures shown below represent plots of vapour pressure V P versus temperature T molecular weight of ethanol is 46 g mol 1 Among the following the option representing change the freezing point is A B V P bar V P bar Ice 270 273 Ice 1 Water Ethanol 1 I T K I I 270 273 Water T K Water Water Ethanol V P bar V P bar Ice Ice I I 1 271 273 T K 1 271 Water I Water Ethanol Water 1 IWater Ethanol 273 T K 2017

Physical Chemistry
Chemical kineticsA definite volume of H O under going spontaneous decomposition required 25 ml of standard permanganate solution for titration After 10 and 20 minutes respectively the volumes of permanganate required were 15 ml and 9 ml respectively Calculate the fraction of H O decomposed after 25 minutes Given 0 6 2 5 0 28 K Xa p 12 21 P q 2

Physical Chemistry
Chemical kineticsFor a reaction AB C it was found that at the end of 10 min from the start the total optical rotation of the system was 50 and when the reaction is complete it was 100 Assuming that only B and C are optically active and dextro rotatory the rate constant of this first order reaction would be a 0 069 min c 6 9 min a b 0 69 min 1 d 6 9 x 10 2 min

Physical Chemistry
Chemical kinetics13 A vessel has 6 g of oxygen at a pressure P and temperature 400 K A small hole is made in it sc that O leaks out How much O leaks out if the pressure is P 2 and temperature 300 K 1 5 g 3 2 g 2 4 g 4 3 g

Physical Chemistry
Chemical kineticsa q is state function in isochoric process b w is state function in isobaric process c Work done by the system will be zero i adiabatic free expansion Correct among the following is are 1 a only 2 b only 3 b c 4 a b c 6

Physical Chemistry
Chemical kinetics4 Number of natural life times T required for a first order reaction to achieve 99 9 leve completion is A 2 3 C 9 2 D 0 105 B 6 9

Physical Chemistry
Chemical kineticsA mixture of methane carbon monoxide Ammonia NO and oxygen The initial volume of gaseous mixture is 130 c c and volume of product gases at similar condition are 60 c c Equal volume of carbon dioxide is produced by combustion of methane and carbon monoxide Volume of O required for combustion of NH3 is 3 5 times 0 require for NO and 7 times of CO Reactions are Not balanced NH3 g O g NO g O g NO g CH g O g CO g H O 1 NO g H O 1 Select incorrect statement 1 Volume of NO and NH3 are same and 20 c c each 2 Volume of CH4 is half of NH3 3 Volume of CO is equal to methane

Physical Chemistry
Chemical kineticsFor the non stoichiometric 2A B C D the following kinetic data were obtained in three different experiments measured at 298 K Initial Conc A 1 3 0 1M 0 1M 0 2M dc dt dc dt Initial Conc B K A B k A B 0 1M 0 2M 0 1M Initial rate of formation of C mol L s 1 2 10 1 2 10 2 4 10 dc 2 k A dt dc 4 K A B dt

Physical Chemistry
Chemical kineticsThe ratio of C 4 to C 2 in a living matter is measured to be 1 C14 C12 Activity of 12 0 gm carbon sample is 180 dpm The half life of C 4 is nearly 10 2 sec Given N 6 x 10 A 0 18 B 1 8 Mrr C 0 384 1 3 x 10 2 at the present time D 648 rith