Electrochemistry Questions and Answers

Physical Chemistry
Electrochemistry3 The equilibrium constant for the reaction Sr s Mg 2 aq Sr 2 aq Mg s is 4 x 10 2 at 25 C The E for a cell made up of the Sr Sr 2 and Mg 2 Mg half cells log 2 0 3 A 0 3717 V B 0 7434 V C 0 1858 V D 0 135

Physical Chemistry
Electrochemistry3 From the following E values of half cells i A e A ED 0 24 V ii B e B E 1 25 V E 1 25 V iv D 2e D E 0 68 V iii C 2e C What combination of two half cells would result in a cell with the largest potential A ii and iii B ii and iv C i and iii D i and iv

Physical Chemistry
Electrochemistryong 2 A metal is known to form fluoride MF When 10A of electricity is passed through a molten salt for 330 sec 1 95g of metal is deposited Find the atomic weight of M What will be the quantity of electricity required to deposit the same mass of Cu from CuSO Colution with inert electrodes for a certain period of time 600 mL of the Was

Physical Chemistry
ElectrochemistryI 2 4 Can t predict 7 The specific conductance of a 0 1 N KCI solution at 23 C is 0 012 ohm cm The resistance of cell containing the solution at the same temperature was found to be 55 ohm The cell constant will be 1 0 142 cm 1 3 0 918 cm 1 2 0 66 cm 1 4 1 12 cm 1 wer Pusa Road New Delhi 110005 Ph 011 4762345

Physical Chemistry
Electrochemistry1 Zn 2 NH 4 NH3 3 MnO The amount of Cu deposited when 10 A of curren is passed for 10 minute through CuSO4 aq is 1 1 97 g 2 2 89 g 3 4 g 4 3 5 g The volume of O g released by 1 C electricity

Physical Chemistry
Electrochemistry2 The standard electrode potential E values of Al AI Ag Ag K K and Cr Cr are 1 66 V 0 80 V 2 93 V and 0 74 V respectively The correc decreasing order of reducing power of the metal is NEET 2019 Odisha 2 Ag Cr AI K 1 Al K Ag Cr

Physical Chemistry
Electrochemistry3 The volume of O g released by 1 C electricity a NTP would be 1 5 6 L 5 6 2 96500 11 2 22 4 3 4 96500 96500 The value of specific conductivity is maximum L L

Physical Chemistry
ElectrochemistryElectrochemistry I Electrolysis of water 3 Questions 1 During electrolysis of water volume ratio of H to O gases obtained is 1 1 2 2 1 3 3 2 1 4 3 1

Physical Chemistry
ElectrochemistryExample 14 The standard reduction potential for the half cell having reaction NO3 aq 2H aq e NO g H O is 0 78 volt i Calculate the reduction potential in 8 M H ii What will be the reduction potential of the half cell in a eutral solution

Physical Chemistry
Electrochemistry1 A galvanic cell is formed by combining Zn rod immersed in Zinc chloride solution of concentration 0 6M and Mg rod immersed in Magnesium chloride solution of concentration 0 15M Write cell representation cell reaction and calculate EMF of cell at 25 C given the Standard reduction potentials of Mg and Zn electrodes as 2 36 and 0 76V respectively

Physical Chemistry
ElectrochemistryAt 298 K the emf of the cell standard hydrogen electrode second half cell constructed by taking standard hydrogen electrode as anode reference half cell and the other half cell as cathode gives the reduction potential of the other half cell If the concentrations of the oxidised and the reduced forms of the species in the right hand half cell are unity then the cell potential is equal to standard electrode potential ER of the given half cell E ER EL

Physical Chemistry
ElectrochemistrySolvelancer Test The formation of Q and R takes place according to given equations The enthalpies of reaction are also given as P Q AH 180 kcal Q R AH 120 kcal The correct order of enthalpies of formation for P Q and Ris Solvelancer Test a P Q R b P R Q c R P Q d Q R P

Physical Chemistry
Electrochemistry0 sho below Pt s 1 A latm 1 A xM B YM IB s The emf measured is 0 30V The cell reaction 1 A 2B 2A 2B 110 2 A 2e 2A 2B 2e2B 3 The cell reaction cannot be predicted 4 2A 2B A 2B

Physical Chemistry
ElectrochemistryWhich of the given statements are incorrect 1 Lime stone is used as flux in the extraction of iron from haematite II Slag separates more easily from ore than the gangue III Froth floatation cannot be used for the benefaction of chalcopyrite IV For zone refining the melting point of the metal should be greater than the impurity O 1 and II only O II and III only O III and IV only O 1 11 and IV only

Physical Chemistry
ElectrochemistryThe resistance of a conductivity cell is 1000 ohm when 0 1 M HA is present in the cell The pH of solution in the cell at 25 degree C is 2 Cell constant and Ka of HA are respectively Given delta M not of HCl NaCl and NaA are 420 126 and 106 S cm2 mol 1 respectively where delta M not is limiting molar conductivity

Physical Chemistry
ElectrochemistryThe molar conductivity of 0 00241 M acetic acid is 32 87 S cm mol If the limiting molar conductivity at 298 K is 390 5 S cm mol what is the dissociation constant mol L 1 1 86 x 10 5 X2 5 36 x 10 5 X3 5 36 x 10 4 X4 1 86 10 4 I

Physical Chemistry
ElectrochemistryKa for the reaction Fe3 aq H O 1 Fe OH 2 aq H3O aq is 6 5x 10 What is the max pH value which could be used so that at least 80 of the total iron III in a dilute solution exists as Fe3

Physical Chemistry
ElectrochemistryThe molar conductance of NaCl HCl and CH3COONa at infinite dilution are 126 45 426 16 and 91 0 S cm mol 1 respectively The molar conductance of CH3CQOH at infinite dilution is Choose the right option for your answer 390 71 S cm mol 1 698 28 S cm 2 mol 1 540 48 S cm mol 1 201 28 S cm2 mol 1 1 2 3 4

Physical Chemistry
ElectrochemistryConsider a 70 efficient hydrogen oxygen fuel cell working under standard conditions at 1 bar and 298 K Its cell reaction is H g 10 H O The work derived from the cell on the consumption of 1 0 x 10 3mol of H g is used to compress 1 00 mol of a monoatomic ideal gas in a thermally insulted containe What is the change in the temperature in K of the ideal gas The standard reduction potentials for the two half cells are given below g 4 H aq 4e 2H O P E 1 23 V 2H aq 2e H g E 0 00V se F 96500 C mol R 8 314 J mol K 1

Physical Chemistry
ElectrochemistryMg2 Mg 0 80 V 0 8 Ag Ag 2 37 V Ecu Cu 0 34 V E EH H8 0 79 V Hg Which of the following statements is correct A AgNO aq can be stored in copper vesse B Cu NO aq can be stored in magnesium vessel C CuCl can be stored in silver vessel D HqC can be stored in copper yessel

Physical Chemistry
ElectrochemistryMolar conductance of Al3 is 150 scm mole and equivalent conductance of SO is 200 scm eq at infinite dilution calculate equivalent conductance of Al SO4 1 225 2 300 3 250 4 290

Physical Chemistry
ElectrochemistryWhen Q amount of electricity passed through aq Cu NO3 6 35 gram of Cu deposited calculate the volume of H liberated when same Q electricity passed through electrolysis of acidified water Atomic weight of Cu 63 5 gr 1 11 2 lit 2 1 12 lit 3 2 4 lit 4 22 4 lit

Physical Chemistry
ElectrochemistryThe CORRECT option s regarding extraction of pure aluminium from red bauxite is are Serpeck s process is used for ore concentration comes as filbrate B Leaching of ore with aq NaOH produces precipitate of NaAlO C Weakly acidic medium is used to precipitate Al OH from solution containing Na Al OH ppt D Only pure Al 0 is used as electrolyte in Hall Heroult s process of electrolytic reduction ugh Work

Physical Chemistry
ElectrochemistryWhich type s of colloids is are correctly matched Multimolecular colloids Gold sols Macromolecular colloids Proteins Associated colloids Soap and detergent All of these

Physical Chemistry
ElectrochemistryColumn l contains some electrochemical reactions and column Il contains their characteristics Column l Q1 2Fe Zn E 0 77 V E Q2 M Ag 0 73 V E 2Fe Zn 20 2 M Ag Ag Ag 0 76 V 0 8V Q3 2H Sn H Sn E S S Q4 Ag Ou Ag Cu E Ag Ag 0 13 V 0 8 V Eriou 0 54 V Column Il A1 E 1 53 V A2 E 0 26 V A3 AG 295290 J A4 AG 25090 J A5 Spontaneous reaction

Physical Chemistry
Electrochemistry48 For the cell reaction 2Ce Co2Ce Co Eo cell is 1 89V and Eco co 0 28 If Ece Ce 1 1 64 V 2 1 64 M 3 2 08 V 4 2 17 V HO 48 les were allowed to react The 49 1 2 3 4 10

Physical Chemistry
ElectrochemistryThe standard reduction potentials for the two half cell reactions are given below Cd2 aq 2e Cd s E 0 40 V Ag aq e Ag s E The standard free energy change for the reaction 2Ag aq Cd s 2Ag s Cd is given by A 115 8 kJ B 115 8 Kj C 231 6 kJ D 231 6 Kj 0 80V

Physical Chemistry
ElectrochemistryIf the hydrogen electrode is dipped in a solution of pH 2 at 25 C then the reduction potential of the electrode would be 0 118 V 0 084 V 0 059 V
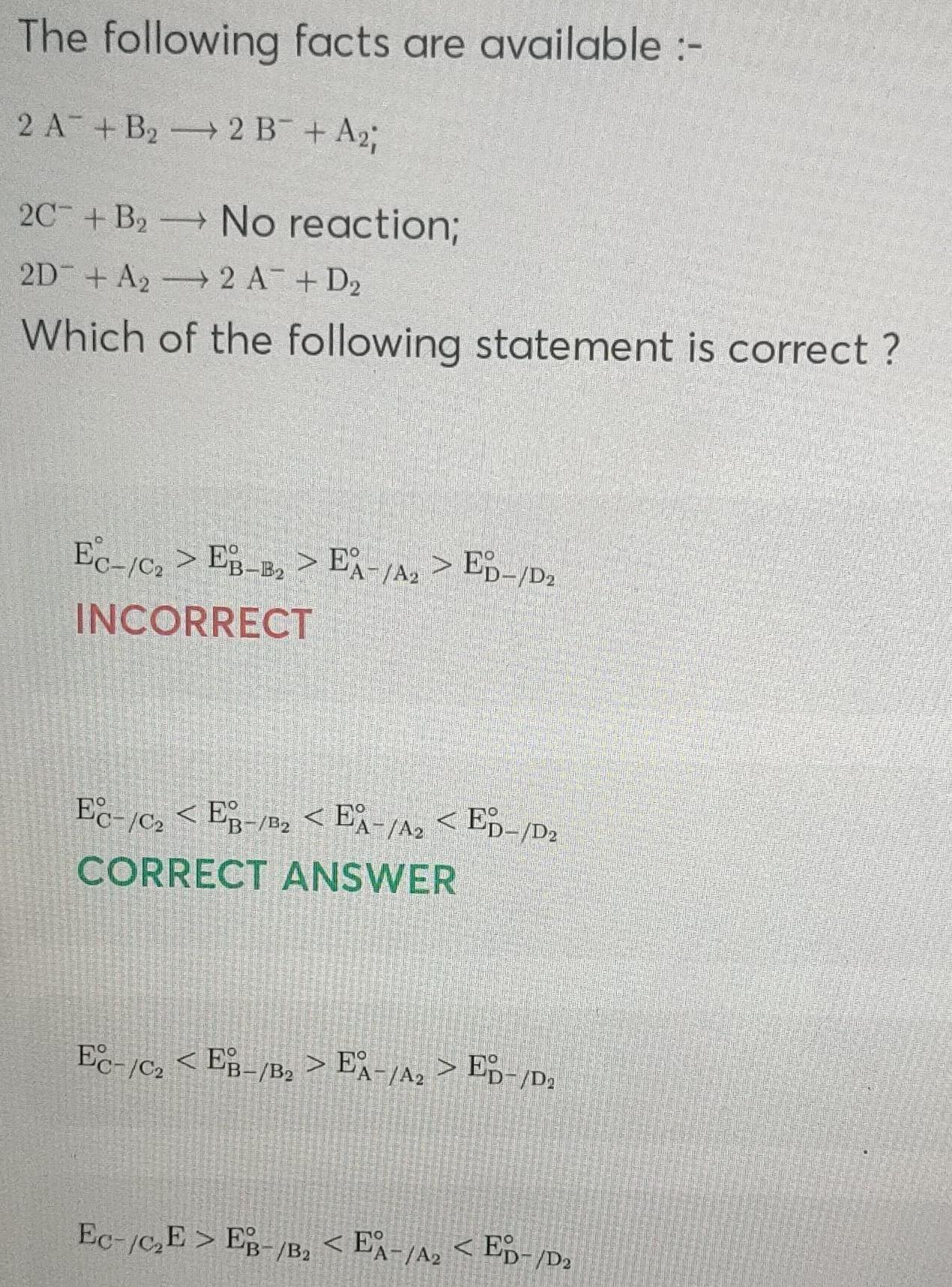
Physical Chemistry
ElectrochemistryThe following facts are available 2 A B 2 B A 2C B No reaction 2D A 2 A D Which of the following statement is correct Ec c EB B EA A ED D INCORRECT EC C2EB B EA A ED D CORRECT ANSWER Ec C EB B EA A ED D Ec C E EB B 2 EA A ED D

Physical Chemistry
ElectrochemistryResistance of a conductivity cell filled with 0 1 mol L KCl solution is 100 Q If the resistance of the same cell when filled with 0 02 mol L KCl solution is 520 2 calculate the conductivity and molar conductivity of 0 02 mol L KCl solution The conductivity of 0 1 mol L KCE solution is 1 29 S m

Physical Chemistry
ElectrochemistryThe following items are obtained from the stockroom for construction of a galvanic cell two beakers and a salt bridge wire with clips 200 mL of a 1 00 M Mn2 aq solution 200 mL of a 1 00 M Au3 aq solution manganese and gold electrodes a Draw a galvanic cell constructed from the items listed above Label the anode and the cathode and show the direction of the electron flow on your drawing

Physical Chemistry
ElectrochemistryThe standard free energy of formation of AgCl s at 25 C is 109 7 kJ mol and H Cl aq is 131 2 kJ mol Find E of a cell made up with standard hydrogen electrode and Cl Ag AgCl s A B C D 0 23 V 0 45 V 0 90 V 0 35 V

Physical Chemistry
ElectrochemistryAn electric current of 0 2F is passed through 2 lit of 0 25M CuSO 4 solution using pt electrodes After electrolysis the molarity of the solution is assume that there is no change in the volume of solution

Physical Chemistry
ElectrochemistryIf 0 224 litre of H gas is formed at the cathode how much O gas is formed at the anode under identical conditions Assume 96500 C as one unit of electricity If cost of electricity of producing x gm Al is Rs x what

Physical Chemistry
Electrochemistry4 4 Which of the following expressions is correct for the molar conductivity of strong electrolytes 1 A m Am A c 2 2 Am A m A c 2 3 Am Am A c 2 4 Am Am A c 2 Ons 1 1 2 2 3 3 Question Type MCQ Question ID 1908891279 Option 1 ID 1908895049 Option 2 ID 1908895050 Option 3 ID 1908895051 Option 4 ID 1908895052 Status Answered Chosen Option 2

Physical Chemistry
Electrochemistry13 A solution containing 1M XSO4 aq and 1M YSO4 aq is electrolysed If conc of X is 10 M when deposition of Y 2 and X2 starts simultaneously calculate the value of Z 2 303RT Given 0 06 F E x2x 0 12 V Ey2y 0 24 V 4 Molar conductivity of aqueous solution of HA is 200 Scm mol pH of this solution is 4 Calculate the value of pK HA at 25 C Given AM NaA 100 Scm mol A HCI 425 Scm mol A NaCl 125 Scm mol M

Physical Chemistry
ElectrochemistryWhich of the following is true about concentration cells 1 Flow of electrons occurs due to difference in concentration of both half cell electrode or electrolyte 2150 3 F 4 Both 1 and 2 are correct

Physical Chemistry
ElectrochemistryWhich of the following reaction will not take place Select an answer A B Zn FeSO4 ZnSO4 Fe D 2KI Cl 2KCl I C Zn MgSO4 ZnSO4 Mg Mg CuSO4 MgSO4 Cu

Physical Chemistry
Electrochemistry78 The concentration of H and concentration of OH of a 0 1 aqueous solution of 2 ionised weak acid is ionic product of water 1 10 41 a 2 10 M and 5 10 2 M b 1 10 M and 3 10 1 M c 0 02 10 M and 5 10 1 M d 3 102 M and 4 10 3 M 1999 9 The solubility of a saturated solution of calcium fluoride is 2 x 10 moles per litra It 3H is a K 85 The ion Its ionic a 1 x c 1 x

Physical Chemistry
ElectrochemistryAgCl Ag can be most easily coagulated by least concentration of O Br O co O PO O Fe CN 4

Physical Chemistry
Electrochemistry6 Mark the correct choice of electrolytes represented in the graph Am S cm mol YO A B C1 2 mol L 1 1 2 a A NH OH B NaCl b A NH OH B NH CI c A CH COOH B CH3COONa 1 NILI

Physical Chemistry
ElectrochemistryWhich of the following does not behave as emulsifying agent O Lampblack O Gold sols O Synthetic soaps Gums

Physical Chemistry
ElectrochemistryWhich one of the following does not hold good for S H E A The pressure of hydrogen gas is 1 5 atmosphere B The concentration of H in solution is 1M C The temperature is 298K D The surface of platinum electrode is coated with platinum black

Physical Chemistry
ElectrochemistryWhich of the following is are correct for potash alum B A alum Aq K SO 2q A1 eq B alum req Ne C alum eq D 1 4 eq q K Al 1 SO 3 4 eq akash eq alum 8 alum K A S0 Founda 00

Physical Chemistry
Electrochemistrylease explain The standard oxidation potential of zinc and silver in water at 298 Kare Zn s Zn 2e E 0 76 V 2 Ag s Ag 2e E 0 80 V IN SUPE Which of the following reactions actually take place NCERT 1983 84 KCET 2003 a Zn s 2Ag aq Zn aq 2 Ag s b c d Zn aq 24g s 24g aq Zn s Zn s Ag s Zn aq Ag aq Zn aq Ag aq Zn s Ag s C

Physical Chemistry
ElectrochemistryHow do we know which reaction is oxidation and which o ne is reduction Please explain 1 Which of the following is the cell reaction that occurs when the following half cells are combined E 0 54 V 1 2e 21 1 M Br 2e 2Br 1 M E 1 09 V a 2Br 1 Br 21 b 1 Br 21 2Br 21 Br 1 2Br d 21 2Br I Br Oxidation Reduction

Physical Chemistry
ElectrochemistryCopper reduces NO into into NO and NO depending upon the concentration of HNO3 in solution Assuming fixed Cu for reduction of NO into NO and NO by copper is same is 10 M The value of 2x is the nearest integer Given E Cu and PNo Pxo the HNO3 concentration at which the thermodynamic tende Rounded of Cu Cu 0 34 V ENO NO 0 96 V ENO NO RT 0 79 V and at 298 K 2 303 0 059

Physical Chemistry
ElectrochemistryThe magnitude of the change in oxidising power of the MnO Mn couple is x x 10 4 V if the H concentration is decreased from 1M to 10 4M at 25 C Assume concentration of MnO4 and Mn to be same on change in H concentration The value of x is Rounded off to the nearest integer Given al 2303R7

Physical Chemistry
ElectrochemistryQ 59 Through an aqueous solution of an unknown salt of metal M M 200 g mol a current of 1 93 A is passed for 50 min If 4 g of metal is produced at cathode The charge on metal ion in solution is

Physical Chemistry
Electrochemistry7 Which of the following arrangement will produce oxygen 57 s at anode during electrolysis 1 Dilute HLSO with Pt electrodes 2 Fused Ned with inert electrodes 3 Dilute HLSO with Cu electrodes 4 Conc aq Nad with Pt electrodes 3