Electrochemistry Questions and Answers

Physical Chemistry
ElectrochemistryA current of 0 1A was passed for 2hr through a solution of cuprocyanide and 0 3745 g of copper was deposited on the cathode Calculate the current efficiency for the copper deposition Cu 63 5 O O O 79 39 5 63 25 62 5

Physical Chemistry
ElectrochemistryGiven that E Ag aq Ag s 0 80 V E Ni aq Ni s 0 25 V the value of E corresponding to the reaction cell 2Ag aq 0 01 M Ni s 2Ag s Ni aq 0 0001M is 2 A 0 991V B 1 05V C 0 55 V D 1 70 V

Physical Chemistry
Electrochemistry3 Atoms consist of electrons protons and neutrons If the mass attributed to neutron was halved and that attributed to the electrons was doubled the atomic mass of 6C 2 would be approximately a same c halved b doubled d reduced by 25 hos the electronic configuration 18 Az

Physical Chemistry
ElectrochemistryStandard electrode potentials are Fe2 Fe E 0 44 volts Fe3 Fe2 E 0 77 volts Fe2 Fe and Fe blocks are kept together the 1 Fe increases 2 Fe decreases 3 Fe2 Fe remains unchanged 4 Fe decreases
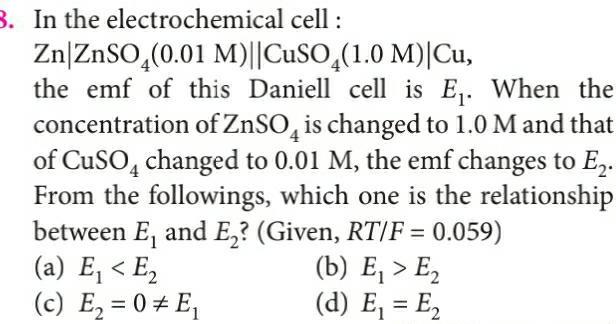
Physical Chemistry
Electrochemistry3 In the electrochemical cell Zn ZnSO4 0 01 M CuSO4 1 0 M Cu the emf of this Daniell cell is E When the concentration of ZnSO4 is changed to 1 0 M and that of CuSO4 changed to 0 01 M the emf changes to E From the followings which one is the relationship between E and E Given RT F 0 059 b E E a E E c E 0 E d E E

Physical Chemistry
ElectrochemistryZn s Porous barrier Zn NINO Cu 1M IM IM Ea Zn Cu s A Galvanic cell consist of three compartment as shown in figure The first compartment contain ZnSO4 1 M and III compartment contain CuSO4 1 M The mid compartment contain NaNO3 1 M Each compartment contain 1 L solution 0 34 0 76 EC Cu Zn 26 The concentration of Zn in first compartment after passage of 0 1 F charge will be b 1 05 M c 1 025 M d 0 5 M 20 2 are will be

Physical Chemistry
ElectrochemistryThe limiting conductivity of NaCl KCI and KBr are 126 5 150 0 and 151 5 S cm eq respectively The limiting equivalent ionic conductance for Br is 78 S cm eq The limiting equivalent ionic conductance for Nat ions would be O 128 O 125 O 49

Physical Chemistry
ElectrochemistryA hydrogen gas electrode is made by dipping platinum wire in a solution of HCl of pH 10 and by passing hydrogen gas around the platinum wire at one atm pressure The oxidation potential of electrode would be a 0 118 V c 0 059 V b 1 18 V d 0 59 V NEET 2013

Physical Chemistry
Electrochemistry1 61 For the cell H pH 1 H pH 2 H 0 1 atm Pt Pt H 0 4atm the measured potential at 25 C is 1 0 025 V 2 0 76 V 3 0 041 V 4 None 62 When H reacts with Na it acts as a 800 700 1500 avo oo 61 fu Pt H 0 4atm H pH 1 H pH 2 H 0 1 atm Pt 25 C 1 0 025 V 3 0 041 V TUELT 2 0 76 V 4 None 62 H H a 2 la 103 ph day 1

Physical Chemistry
Electrochemistry10 ampere current is passed through CuSO4 solution for 193 minute at 1 bar pressure and 300 K temperature If cell has 80 capacity then find out weight of Cu and volume of 0 Cu 65 3 u R 0 08314

Physical Chemistry
Electrochemistry113 Given E O 2 1 51 V 1 36 V Cr Cr 0 74 V EMnO4 Mn Cr 0 7 Cr3 1 33 V ECI CI Based on the data given above the strongest oxidising agent will be a Mn 2 c CI b MnO4 3 d Cr

Physical Chemistry
Electrochemistry3 0 78 V 4 0 34 V 3 A graph was plotted between molar conductivity of various electrolytes NaCl HCI and NH OH and C in mol L Correct setting Molar conductivity C III 1 I NaCl II HCL III NH OH 2 I HCI II NaCl III NH OH 3 I NN OH II NaCl III HCI 4 I NH OH II HCL III NaCl 87 88

Physical Chemistry
Electrochemistry70 Given EAg Ag 0 80V EMg Mg 2 37V 0 34V EHg Hg ZENGER Cu Cu statement is correct 0 79V Which of the following 1 AgNO can be stored in copper Vessel 2 Mg NO3 can not be stored in copper vessel 0804 2 3 CuCl can be stored in copper vessel 4 HgCl can be stored in copper vessel 70 nc E Mg Mg 237V Ag Ag 0 34V Eg Hg 0 79V FAM D E TY 1 E Cu Cu 464 20 3 1 AgNO face 2 Mg NO face 0 80V E Sex Cuci Cuci a factors and forest in an interna 3 CuCl 4 HgCl faccia 2 Handy 1 X

Physical Chemistry
ElectrochemistryS 12 6 4 None 4 None 55 The EMF of the cell Cr Cr 0 1 M Fe 2 0 01 M F 55 f 12 Given E 3 cr 0 75 V Efe Fe 0 45 V 2 Cr3 Cr 1 0 26 V 2 0 52 V 3 0 72 V 4 0 60 V Be show diagonal rola 754 45 36 he ALI M log x 4x102 MAIL 76 Cr Cr 0 1 M Fe 0 01 M Fe fydd Q Fe Fea EC Fe 2 Fe 0 45 V 0 75 V C Te Feed N Fe ost by LI fl 1 0 26 V 2 0 52 V 3 0 72 V 4 0 60 V 3 Cr 4 Fe G 30 10

Physical Chemistry
Electrochemistry4 I NH OH II HCL III NaCl 4 The specific conductance of a saturated solution of silver bromide is k Scm The limiting ionic conductiveity of Ag and Br ions are x and y respectively The solubility of silver bromide in gL is molar mass of AgBr 188 1 3 xx1000 x y x1000x188 x y 2 K x y 188 x y 1000 K 188 tr

Physical Chemistry
ElectrochemistryEach of the three metals X Y and Z were put in turn into aqueous solution of the other two X Salt of Y or Z Y or Z Salt of X Which observation is probably Incorrect 0 A B Y Salt of X No action observed Your Answer Y Salt of Z Z Salt of Y CZ Salt of X X Salt of Z Correct Answer Z Salt of Y No action observed

Physical Chemistry
Electrochemistryif 10 What is the potential of the cell containing two hydrogen electrodes as represented below Pt H g H 10 M H 0 001 M H g Pt 1 0 295 V 13 0 295 V 2 0 0591 V 4 0 0591 V col

Physical Chemistry
Electrochemistryc CIT 114 Given below are the half cell reactions VMn 2e Mn E 1 18 V 2 Mn 3 e Mn Mn 2 E 1 51 V The E for 3Mn 2 Mn 2Mn 3 will be a 2 69 V the reaction will not occur b 2 69 V the reaction will occur c 0 33 V the reaction will not occur d 0 33 V the reaction will occur

Physical Chemistry
Electrochemistryb Fe 1 Fe s Above equilibrium is favoured at High pressure and low temprature 2 High pressure and high temperature 3 Low pressure and high temperature 4 Low pressure and low temperature 7 For the reaction N g O g 2NO g PEDRY 46 Fe 1 Fe s 1 2 3 4 Chemical fff Equilibrium 37N g O g 2NO g

Physical Chemistry
Electrochemistry5 A concentration cell is constructed by dipping copper rod in 0 002M and 0 2M copper sulphate solution respectively Write cell representation cell reaction and calculate EMF of cell

Physical Chemistry
Electrochemistrya og c 2 g 4 116 For the following electrochemical cell at 298 K Pt s H g 1 bar H aq 1 M M aq M Ecell 0 092 V when M aq 10 2 M4 aq 03 RT F Given E4 M2 0 151 V 2 303 The value of x is a 2 c 1 b 1 d 2 aq Pt s 0 059 V

Physical Chemistry
Electrochemistry1 mole of electrons are transferred during electrolysis consider only electrolysis of water in both compartments pKa pKa values of H CO3 are x y respectively pH of anode cathode respectively after electrolysis SRAMA EVA AYATHE 1 lit solution of Na CO3 NaHCO3 1 lit solution of Na CO3 NaHCO3

Physical Chemistry
Electrochemistry60 Calculate Am of oxalic acid given that Aeq Na C O 400 2 cm eq 0 Am H SO4 700 cm mole 8 Aeq Na SO 4502 cm eq 1 400S cm mol 2 600S cm mol 3 800S cm mol 4 None Forth 0 crof Cr o O Komnou ni 2 MP Mn IND 6 2 1 5

Physical Chemistry
ElectrochemistryTl s Tl 0 0001 M Cu 0 01M Cu s 0 83 V The emf of this cell will be increased by 1 Increasing the concentration of Cu 2 ions 2 Decreasing the concentration of Tl 3 Increasing the concentration of both 40 1 2 both

Physical Chemistry
ElectrochemistryDuring the electrolysis of sodium chloride in Castner Kellner cell the product obtained at cathode and anode respectively are O Na Cl O H Cl2 O Na amalgam Cl

Physical Chemistry
Electrochemistry4 H Ni 2H Ni The following facts are available 2X Y 2Y X 2W Y no reaction 2Z X 2X Z Which of the following statements is correct 1 Eo 42 E 3 Eo 141 Do W W E y Y E x x E z Y E w W E y Y Ex x E z 2 Y Y X X2 2 22 W W E y Y E x x E Y X X2 2 22 Co Fo 2 22 Fo 5

Physical Chemistry
Electrochemistry100 The specific conductance of a saturated solution of silver bromide is k S cm The limiting ionic conductivity of Ag and Br ions are x and y respectively The solubility of silver bromide in gl is molar mass of AgBr 188 Ans kx1000 188 x y Sol Solublity Ac x y Solubility 1000 specific conductance Ac 1000 k x y x188 g L

Physical Chemistry
ElectrochemistryThe specific conductance of a salt of 0 01 M concentration is 1 061 x 10 4 S cm Molar conductance of the same solution will be 1 1 061 x 104 3 10 61 2 1 061 4 106 1 14 The specific KCI is 0 001 conductance 1 140 3 1 4

Physical Chemistry
Electrochemistry38 Adding powdered Pb and Fe to a solution containing 1 0 M in each of Pb 2 and Fe 2 ions would result into the formation of 1 More of Fe and Pb2 ions 2 More of Fe 2 and Pb ions 3 More of Pb and Fe 2 ions 4 More of Fe and Pb

Physical Chemistry
Electrochemistry4 ve ve ve 17 Given the standard electrode pontentials K K 2 93 V Ag 0 80 V Hg2 Hg 0 79V Mg2 Mg 2 37 V Cr2 Cr Arrange these metals in their increasing order of reducing 0 74 V 17 EP 4 ve ve ve K K 2 93 V Ag 0 80 V Hg2 Hg 0 79V Mg2 Mg 2 37 V Cr2 Cr 0 74 V dan KT ng power agen 1 Ag Hg Cr Mg K 2 Ag Hg Mg Cr K 1 Ag Hg Cr Mg K 2 Ag Hg Mg Cr K 3 Mg K Ag Hg Cr 3 Mg K Ag Hg Cr 4 Ag Mg Hg Cr K 4 Ag Mg Hg Cr K The spntaneous redox reaction s among the following is 18 fe fall ad it 12 x cr

Physical Chemistry
Electrochemistry03 The rusting of iron takes place as follows d 5 49x10 0 C H O 1 H O 1 E 1 23 V 2e Fe s E 0 44 V The AG for the net process is a 322 kJ mol 1 c 152 kJ mol 1 Given the data at 25 C 2H 2e 2e 10 Fe 2 b 161kJ mol d 76 kJ mol 1

Physical Chemistry
ElectrochemistryThe resistance of M 10 solution of an electrolyte is 250 ohm The cell constant is 1 25 cm Then the molar conductance of the solution is s cm mol 1 100 2 125 3 250 4 50

Physical Chemistry
ElectrochemistryDuring the electrolysis of aqueous K SO4 using inert electrodes to produce 0 1 mole of oxygen gas at anode using a current of 5 amp the time required is seconds 11 3860 2 1930 3 7720 4 17232

Physical Chemistry
ElectrochemistryWhich of the following equation represents the standard heat of formation A C diamond 2H g CH g B C graphite 2H g CH g C C diamond 4H g CH g D C graphite 4H g CH g

Physical Chemistry
ElectrochemistryWrite the formulation for the galvanic cell in which the reaction Cu s 2Ag aq Cu aq 2 Ag s takes place Identify the cathode and anode reactions in it 1 Write Nernst equation and calculate the e m f of the following cell Sn s Sn 0 04 M H 0 02 MOH

Physical Chemistry
ElectrochemistrySame quantity of electricity is passed through molten NaCl MgCl2 and AIC connected in series Then the ratio of the volumes of chlorine gas liberated fro these three electrolytes respectively is 1 1 2 3 2 3 2 1 3 1 1 1 4 6 3 2

Physical Chemistry
Electrochemistry47 At 25 C Ag NH3 e Ag s 2NH E 0 02V Ag e Ag s E 0 8V 3 9 What will be the range of magnitude of equilibrium constant of following reaction 6 3 X100p 63X250 1 40 XIX Ag NH3 Ag 2NH 1 10 14 2 106 3 10 10 4 10 18 XFXE OU 4 0 94 volt 47 25 C Ag NH3 e Ag s 2NH3 E 0 02V Ag s E 0 8V Ag di alfafed fefe B 1 x y 20 Ag NH 1 10 14 XXXXood 1006 2 106 1X X018 3 10 10 4 10 18 Ag 2NH feric 0178 0106 lug koq 78 13 0 8 78 10

Physical Chemistry
ElectrochemistryThe electrochemical equivalent of a metal is 9 326 x 105 g coulomb If 30 amp current is passed through the molten metal chloride for 10 minutes then the weight of the metal deposited is g 1 0 028 2 0 056 3 1 68 4 3 36

Physical Chemistry
Electrochemistry4 5 mL 2 sec 61 The reaction 10 7 1 H g AgCl s H aq Cl aq Ag s 2 Occurs in the galvanic cell 1 Ag AgCl s KCI sol AgNO3 sol Ag 2 Pt H g HCl sol AgNO3 sol Ag 3 Pt H g HCl sol AgCl s Ag 4 Pt H g KCI solr AgCl s Ag 3216 2 3 100 mL 10 sec 4 5 mL 2 sec 61 3 1 is 7 8 X t L H g AgCl s H aq CF aq Ag s fa af 1 Ag AgCl s KCI sol AgNO3 sol Ag 2 Pt H g HCl solr AgNO3 sol Ag 3 Pt H g HCl sol AgCl s Ag 4 Pt H g Kel sol AgCl s Ag X 10 2 x 5 3 1 FC

Physical Chemistry
ElectrochemistryIf a galvanic cell is represented as Mg Mg 1M Fe 1M Fe s Then the reaction takes place at right hand half cell is 1 Mg s Mg2 aq 2e 3 Fe s Fe aq 2e 2 Fe aq 2e 4 Mg aq 2e Fe s Mgs

Physical Chemistry
Electrochemistry6 02 x 1024 electrons are passed through aqueous CuSO solution using iner electrodes Then the weight of copper deposited at cathode is 8 AW 63 5 1 635 2 317 5 3 63 5 4 31 75

Physical Chemistry
ElectrochemistryThe conductivity of 0 02 M a weak acid HX is 8 x 10 conductances of H and X ions are 350 and 50 Then the degree of dissociation of HX under these experimental conditions is S cm The limiting molar ohm cm mol respectively 1 0 156 2 0 24 3 0 065 4 0 1

Physical Chemistry
Electrochemistry50ml of 0 1M CUSO4 solution is electrolysed by using pt electrodes with a current of 0 965 ampere for a period of one minute Assume volume of solution does not change during electrolysis 33 Concentration of H after Jootrolvain in

Physical Chemistry
Electrochemistryde potential for the reaction Ag aq e Ag s Sn aq 2e Sn s at 25 C are 0 80 volt and 0 14 volt respectively The emf of the cell Sn Sn 1M Ag 1M Ag is 1 0 66 volt 2 0 80 volt 3 1 08 volt 4 0 94 volt At 25 C Ag NH3 e Ag s 2NH E 0 02V 46 25 C Ag aq e Ag s Sn aq 2e Sn s af 0 80 Sn Sn 1M Agt 1M Ag Oxi 1 0 66 volt 2 0 80 volt 3 1 08 volt 4 0 94 volt 47 25 C T PCB 7 to 9 0 14are EMF 0 80 8 79 0

Physical Chemistry
ElectrochemistryEAIA B B Ec 0 3V Ep 0 1V D D Based on above data select correct statement under standard condition Best oxidising agent is A Best reducing agent is D C can displace D from it s solution In galvanic cell obtained by electrodes A B current flow from B to A in external circuit 1

Physical Chemistry
ElectrochemistryThe rechargeable cells used in calculators and battery operated tools are based on nickel and cadmium electrodes It may involve which of the following cell reactions to make cell reaction spontaneous Cd s 20H aq Cd OH 2e E 0 40 V OXI Ca OH 2e Cd s 2OH aq E 0 40 V 2Ni OH s 20H aq 2NiO OH s 2H O l 2e E 0 49 V 2NiO OH s 2H O 1 2e 2Ni OH s 2OH aq Ed 0 49 V

Physical Chemistry
Electrochemistry2 What will be standard cell potential of galvanic cell with the following reaction 2Cr s 3Cd aq 2Cr aq 3Cd s Given E C Cr 0 74 V and E Ca Ca 0 40 V a 0 74 V b 1 14 V c 0 34 V d 0 34 V

Physical Chemistry
ElectrochemistryPbl4 does not exist because Question Type Single Correct Type 1 lodine is not reactive 2 Pb IV is oxidizing and I is reducing agent 3 Pb IV is less stable than Pb II 4 Ph 4 is not easily formed

Physical Chemistry
Electrochemistry29 Given below are few reactions with some expressions Mark the expression which is not correctly matched a For concentration cell Ag Ag C Ag C Ag 0 0591 C Ecell 1 C2 b For the cell 2Ag H 1 atm 2Ag 2H 1 M 0 0591 Ecell Ecell 2 12 Ag 1 H 1 c For an electrochemical reaction at equilibrium aA bB 0 0591 log Excell ne n d For the cell M 0 0591 E E log CC dD C D d A B n aq log ne M s 1 log

Physical Chemistry
Electrochemistry03 Be and a proton are accelerated by the same potential their de Broglie wavelengths have the ratio assume mass of proton mass of neutron 1 1 2 2 1 4 3 1 1 4 1 3 3