Equilibrium Questions and Answers

Physical Chemistry
Equilibriumc Between 8 and 9 d Between 6 and 7 8 The pH of a dilute solution of acetic acid was found to be 4 3 The addition of a small crystal of sodium acetate will cause pH to a Become less than 4 3 b Become more than 4 3 c Remains equal to 4 3 d Unpredictable

Physical Chemistry
EquilibriumThe molar ratio of sodium acetate to acetic acid in a buffer solution with a pH of 5 76 is 5 1 Assuming the total buffer concentration is 2 2 x 102 mol L how many grams of sodium acetate m w 82 should be used in preparing a liter of the solution Round answer to the nearest hundredth Do not include units

Physical Chemistry
Equilibrium15 At 600 C K for the following reaction is 1 atm P X g Y g Z g at equilibrium 50 of X g is dissociated The total pressure of the equilibrium system is P atm What is the partial pressure in atm of X g at equilibrium a 1 b 4 c 2 d 0 5

Physical Chemistry
Equilibriumc 9 12 For the reaction AB g A g B g AB is 33 dissociated at a total pressure P Therefore P is related to K by which of following options P b P 3K d P 8K a P K c P 4K P d 1 6 P 3 2HI g IT P 1 3

Physical Chemistry
Equilibrium22 The standard Gibbs energy change at 300 K for the B C is 2494 2 J mol At a given time the composition of the reaction mixture is reaction 2A A M B 2M C M The reaction 2 2 proceeds in the R 8 34 J k mol e 2 718 a Forward direction because Q K b Reverse direction because Q K c Forward direction because Q K d Reverse direction because K

Physical Chemistry
Equilibriumhe degree of hydrolysis of salt of weak acid and weak base in its 0 1M solution is found to be 50 If the molarity of th olution of same salt is 0 5M then the percentage degree of hydrolysis of the salt should be 1 2 3 100 50 25

Physical Chemistry
Equilibriumplain which combination is the best choice to prep a buffer with a pH of 9 0 a NH3 NH4Cl pKb for NH3 4 75 b C5H5N C5H5NHCI pKb for C5H5N 8 76 c HNO2 NaNO2 pKa for HNO2 3 33 d HCHO2 NaCHO2 pKa for HCHO2 3 74

Physical Chemistry
EquilibriumWhat is the concentration of Ba CN 2 in the aqueous solution of the mixture of Ba CN 2 and 0 1 M HCN having pH 5 Given pkb for CN at 25 C is 8 2 3 4 10 2 M 102 M 2x 10 2 M 5 x 10 3 M

Physical Chemistry
Equilibrium3 Eight mole of chlorine Cl undergoes a loss and gain of 14 mole of electrons to form two oxidation states of chlorine CI Write down the two half reactions and equation for disproportionate of chlorine Cl

Physical Chemistry
EquilibriumA 1 025 g sample containing a weak acid HX molecular weight 82 g mot is dissolved in 60 mL water and titrated with 0 25 M NaOH When half of the acid was neutralised the pH was found to be 5 0 At the equivalence point the pH was 9 0 The weight percentage purity of HX in the sample is Correct Answer 80

Physical Chemistry
EquilibriumQ Please help me with the following MC Thnx 1 When solid aluminum sulfate is in equilibrium with its ions the ratio of aluminum ions to sulfate ions is a 1 1 d 2 1 b 1 2 e 2 3 c 1 3 f 3 1 2 Which of the following cannot act as a Brons

Physical Chemistry
Equilibrium15 The equivalent conductivity of KCl at infinite dilution is 130 mho cm eq The transport number of Clion in 2 KCl at the same temperature is 0 505 The transport number of K ion is La 0 495 4 x 1 1 cl tk c 0 0495 b 0 505 d cannot be predicted In the problem 15 ionic conductance of K ion is a 64 35 b 60 20 c 262 26 d 26 22

Physical Chemistry
EquilibriumFor the equilibrium N2 g 3H g 2NH3 g the equilibrium constant K is expressed as Kp A B Solution Kp D C Kp Kp 3 PN2 PH Kp N2 3H22NH3 NH PN2 Kp X 2 P NH3 PNH3 3 P N P NH3 3 PN PH PN X 3pN PNH P NH3 4 3 X PN2 P NH3 4 33 X PN2 P NH3 3 32 3 P N2 4 X PN2 3PN PH

Physical Chemistry
EquilibriumThe solubility product of AgCl is 1 8x10 Precepitation of AgCl will occur only when eq volumes of which of the following solutions are mixed 1 10 M Ag and 10 M CI 2 10 M Ag and 10 MCI 4 10 M Ag and 10 M CI 3 10 M Ag and 10 M CI

Physical Chemistry
EquilibriumAn empty steel vessel is charged with 0 430 atm of C and O 430 atm of A Once the system reaches equilibrium according to the reaction below what is the equilibrium partial pressure of C Kp for this reaction is 11 2 A g B g C g

Physical Chemistry
EquilibriumCH3COOC H5 H 0 CH3COOH C H5OH Ans 4 17 75 gram of PC15 is heated in a closed vessel of one litre at 580 K Chlorine formed at equilibrium is 3 337 g Calculate K for the reaction PC15 8 Cl g PC13 g Ans 0 058 mol L hemical equilibrium reaction

Physical Chemistry
EquilibriumIn the heterogeneous chemical equilibrium reaction NH4 2S g 2NH3 g H S g the total pressure of the system at constant temperature and at equilibrium is 1 2 atmosphere Calculate Kp for the reaction Ans 0 256 atm un moles of PC are heated at 600 K in a closed vessel of 2 litre At equilibrium 40 PC15 PCL Clofg

Physical Chemistry
Equilibrium100 ML of 1 2M FeCl3 solution is mixed with 200ml of 1 5M Mg Cl and the resulting solution is dilute to 500ml Then FeCl Fe 3Cl MgCl Mg 2CH A The molarity of Fe in the resulting solution is 0 24 M B The molarity of Mg2 ion in the resulting solution is 0 6M C The molarity of CI D The molarity of CI ion in the resulting solution is 1 92 M ion in the resulting solution is 3 2 M

Physical Chemistry
Equilibrium8 For the reversible equilibrium reaction A 2B 3C 4D the rate constant for the forward and backward reaction are 8 15 x 10 5 respectively Calculate K sible reaction e 2 38 x 10 and Ans 2 920

Physical Chemistry
EquilibriumAt 525 K PC15 g is 80 dissociated at a pressure of 1 atm Now sufficient quantity of an inert gas at constant pressure is introduced into the above reaction mixture to produce inert gas partial pressure of 0 9 atm What is the percentage dissociation of PC1 g when equilibrium is re established a 97 3 c 65 6 b 80 d 4 7

Physical Chemistry
Equilibrium1 For the reaction given below a 1 0 L container initially contained 3 0 mol L of A g and 2 0 mol L of B g 2A g B g C g At equilibrium the concentration of C g is 0 75 mol L What is the concentration of A g

Physical Chemistry
Equilibrium25 Equal volumes of 10 v v of HCl is mixed with 10 v v NaOH solution If density of pure NaOH D can t be predicted 1 5 times that of pure HCI then the resultant solution be A basic B neutral C acidic

Physical Chemistry
Equilibriumindicate whether solutions with each of the following ion concentrations are neutral acidic or basic at 25 C Note that 1 means acidic 1 means basic and 0 means neutral a OH 1 68 10 10 M b H 7 94 x 10 10M c H 1 10 M d H 4 19 10 M e OH 9 73 10 22 M

Physical Chemistry
EquilibriumPotassium sorbate C6H7KO2 is an additive used to prevent molding of cheese Its conjugate acid monoprotic has Ka 1 74x10 5 Find the pH of the aqueous solution containing 2 55 g of potassium sorbate in 500 0 mL

Physical Chemistry
EquilibriumIf the concentration of OH ions in the reaction Fe OH 3 s Fe aq 3OH aq is 1 4 concentration of Fe3 will increase by decreased by 1 4 times 3 16 times times then equilibrium AIPMT Prelims 2008 2 8 times 4 64 times

Physical Chemistry
EquilibriumCalculate the hydronium ion concentration and the pH at the equivalence point in a titration of 50 0 mL of 0 040 M NH with 0 04 MHCI K 1 8x10 Please don t paste a solution from inter net I already looked at them and they h ave incorrect solutions I solved it by finding concentration of N H4Cl at equivalnce point 2 10 3 and found the pH using hydrolysis formula f or SAWB and the pH came as 5 97 but t

Physical Chemistry
EquilibriumThe ionization constant of ammonium hydroxide is 1 77 x 10 5 at 298 K Hydrolysis constant of ammonium chloride is AIPMT Prelims 2009 1 6 50 10 12 3 5 65 x 10 12 2 5 65 x 10 13 4 5 65 10 10

Physical Chemistry
Equilibrium141 What is the molar solubility of Ag 2CO3 Ksp 4 x 10 13 in 0 1 M Na CO3 solution b 10 7 c 2x 10 6 a 10 6 142 What is the concentration of Pb 2 when PbSO4 Ksp 1 8 10 8 begins to precipitate fro d 2x 10 7 solution that is 0 0045 M in SO 2

Physical Chemistry
EquilibriumThe equilibrium A g 4B g AB g is attained by mixing equal moles of A Then at equilibrium 1 A B 3 A B 2 A B 4 AB A a one litre
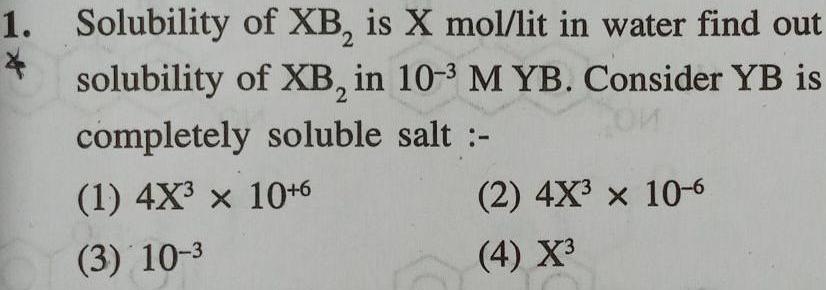
Physical Chemistry
Equilibrium1 Solubility 4 of XB is X mol lit in water find out solubility of XB in 10 3 M YB Consider YB is completely soluble salt 1 4X x 10 6 3 10 3 2 4X x 10 6 4 X

Physical Chemistry
EquilibriumFor the reaction N O g 2NO g 1 2O g calculate the mole fraction of N O g decomposed at a constant volume temperature if the initial pressure is 600 mm Hg the pressure at any time is 960 mm Hg Assume ideal gas behaviour If answer is x then report 10x

Physical Chemistry
Equilibriumd 3 0 10 7 is x and that in 0 1 M AgNO3 is y then which of the following is correct a x y b x y c x y d We cannot predict 145 What is the molarity of Fe CN in a saturated solution of Ag 4 Fe CN 6 Ksp 1 6 10 41 a 1 6 x 10 8 d 2 3 x 10 9 4 b 5 2 x 10 8 c 2 0 10 8 146 At 25 C Ksp for PbBr2 is equal to 8 x 10 5 If the salt is 80 din

Physical Chemistry
EquilibriumAn analytical chemist is titrating 189 8 mL of a 1 200M solution of hydrezoic acid HN with a 1 100M solution of KOH The pX of hydrazoic acid is 4 32 Calculate the pH of the acic solution after the chemist has added 89 21 mil of the KOH solution to it Note for advanced students you may assume the final volume equas the initial volume of the salution plus the volume of KOH solution added Round your answer to 2 decimal places 1 0 pH 5

Physical Chemistry
Equilibrium147 What is the molar solubility of Mn OH 2 Ksp 4 5 x 10 4 in a buffer solution containing equal amounts of NH4 and NH3 Kb 1 8 10 5 a 3 0 x 10 4 b 1 38 x 10 4 48 Find moles of NILL 3 c 1 38 x 10 d 7 3 x 10

Physical Chemistry
Equilibrium0 3 Then find out mole fraction of PCI 1 0 3 2 0 7 3 0 4 4 0 6 If 8 mol of PCl heated in a closed vessel of 10 L capacity and 25 of its dissociates into PCl3 and Cl at the equilibrium then value of Kp will be equal to 1 P 30 2 P 15 3 2 3P 4 3 2P In the reaction PCl5 PCl3 Cl the partial pressure of PCl3 Cl and PCI are 0 3 0 2 and 0 6 atm respectively at equilibrium If partial

Physical Chemistry
EquilibriumConsider the reaction below Which of the following would increase the partial pressure of B at equilibrium A s B g C g AH 0 A adding A B increasing the total volume of the container C increasing the temperature D removing C
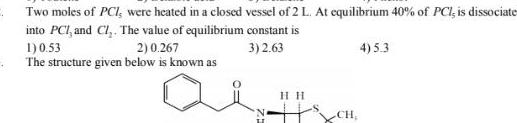
Physical Chemistry
EquilibriumTwo moles of PCI were heated in a closed vessel of 2 L At equilibrium 40 of PCI is dissociate into PCI and Cl The value of equilibrium constant is 3 2 63 1 0 53 2 0 267 The structure given below is known as au HIH CH 4 5 3

Physical Chemistry
EquilibriumFind the concentration of manomeric dichloroacetic acid in a CCl4 solution which contains 0 0129 g of the acid in 100 ml of solution The dissociation constant of the dimeric acid is 5 0 10 4 Assume that the acids are unionized in CCl4 solution a 5 0 x 104 M c 1 0 10 M b 2 5 x 104 M d 3 9 x 104 M

Physical Chemistry
Equilibrium20 Solubility products of M OH and M OH are 10 23 and 10 14 respectively Which will be precipitated first on adding NH OH if M 2 and M 3 both the ions are in solution 2 M 3 4 Precipitation will not take place 1 M 2 3 Both M 2 and M together Ans 2

Physical Chemistry
Equilibriumc 1000 ppm d 1 0 gm of pure calcium carbonate was found to require 50 ml of dilute HCl for complete reaction The strength of the HCI solution is given by CPMT 1986 a 4 N c 0 4 N b 2 N d 0 2 N of an

Physical Chemistry
Equilibriumn analytical chemist is titrating 194 2 ml of a 0 4800M solution of piperidine C H NH with a 0 6500M solution of HIO The pK of piperidine is 2 89 alculate the pH of the base solution after the chemist has added 35 2 ml of the HIO solution to it ote for advanced students you may assume the final volume equals the initial volume of the solution plus the volume of HIO solution added ound your answer to 2 decimal places

Physical Chemistry
EquilibriumWhich of the following is not true for solid liquid equili 1 It can be established at any given temperature 2 The mass of solid does not change with time 3 The mass of liquid does not change with time There is no exchange of heat between the system and its surroundin

Physical Chemistry
Equilibriumven 2 er The solubility of CdSO in water is 8 0 x 10 4 mol L 1 Its solubility in 0 01 M H SO4 solution x10 6 mol L 1 Round off to the Nearest Integer is Assume that solubility is much less than 0 01 M

Physical Chemistry
Equilibrium17 Some chemists at ISRO wished to prepare a saturated solution of a silver compound and they wanted it to have the highest concentration of silver ion possible Which of the following compounds would they use sp K AgCl 1 8 10 10 K AgBr 5 0 10 13 Kp Ag CrO4 2 4x 10 2 a AgCl c Ag CrO 4 b AgBr d Any of them

Physical Chemistry
EquilibriumThe dissociation equilibrium of a gas AB2 can be represented as The degree of dissociation is x and is small compared to 1 The expression relating the degree of dissociation x with equilibrium constant Kp and total pressure p is 1 2KP p 2 2Kp p 3 3 2KP p 1 2 4 KP P

Physical Chemistry
Equilibrium1 1 At a constant temperature Ke for the reaction H g 12 g 2HI is 48 2 Predict the direction in which the reaction will proceed at the same temperature when moles of H I2 and HI present are 1 10 2 2 10 2 and 4 x 10 2 respectively in 1 5 litre vessel Ans forward direction

Physical Chemistry
EquilibriumIn a mixture of equimolar solutions of NaHCO and NaOH the species present in solution shall be 1 Na CO3 3 NaOH 2 NaHCO3 NaOH 4 NaHCO Na C IE03

Physical Chemistry
EquilibriumAn indicator used in a metal EDTA titration is a diprotic acid H A with pK values of pk 7 0 and pk 2 12 0 In a solution buffered at pH 11 the most predominant species of this indicator is 42 OH A O equal amount of H A and HA HA equal amount of HA and A 2

Physical Chemistry
EquilibriumThe value of AH for the reaction X g 4Y g 2XY g is less than zero Formation of XY g will be favored at 1 high temperature and high pressure 2 low pressure and low temperature 3 high temperature and low pressure 4 high pressure and low temperature Which one of the following undergoes reaction with 50 sodium hydroxide solution to giv

Physical Chemistry
EquilibriumArrange them in order of increasing energy from the lowest to highest AAJ KA TOPPER 1 ii iv i 11 2 i iii ii iv 4 iv ii iii 1 3 iii i iv ii pH of 0 1 M BOH weak base is found to be 12 The solution at temperature T K will display on osmotic pressure equal to 1 0 01 RT 2 0 01 RT 3 0 11 RT 4 1 1 RT The artificial sweetener that has the highest sweetness value in comparison to cane sugar is