Equilibrium Questions and Answers

Physical Chemistry
EquilibriumA solution of NaOH is 4 g L 1 What volume of HCI gas at STP will neutralize 50 ml of the alkali solution 1 224 ml 3 11200 ml 2 112 ml 4 22 4 ml

Physical Chemistry
EquilibriumKO H O KOH H O O 28 4 g KO when treated with excess H O gives only 0 34 g H O according to the above reaction Determine the yield of H O KO H O KOH H O O 28 4 g KO at 46 curica 3 0 34 g H O fa si 2

Physical Chemistry
EquilibriumVE 5 What indicatro should be used for the titration of 0 10M KH BO with 1 10 MHCI

Physical Chemistry
EquilibriumVapour density of PCI is 104 16 but when heated to 230 C its vapour density is reduced to 62 The degree of dissociation of PCI at this temperature will be 1 6 8 3 46 2 68 4 64 10225

Physical Chemistry
EquilibriumEquations A K Q B AGO RT In Q ii Equilibrium C K Q iii Spontaneous 869 6 Type of processes i Non spontaneous D T AH ve iv Spontaneous 869 6 AS a A i B ii C iii D iv b A iii B iv C ii D i A iv B i C ii D iii d A ii B i C iv D iii and endothermic T more mey

Physical Chemistry
Equilibrium7 CE0138 In a reaction equilibrium proceeds towards reactants then K will be 1 K 1 3 K 0 2 K 1 4 K 1 CE013

Physical Chemistry
EquilibriumA buffer of pH 9 26 is made by dissolving x moles of ammonium sulphate and 0 1 mole of ammonia into 100 mL solution If pK of ammonia is 4 74 calculate value of x b NH4h Sou s2Nhat so

Physical Chemistry
Equilibrium21C1 g 12 g Cl2 g Equilibrium constant K for given reaction is 0 14 If initally 0 6M ICI was taken then what will equilibrium concentration of I is 1 0 1275 M 2 0 3250 M 3 0 35 M 4 0 2550 M

Physical Chemistry
EquilibriumAt a certain temperature solids NH HS and NH COONH are introduced into an evacuated vessel Consider the dissociation equilibrium of the species NH COONH s 2NH g CO 9 Kp Kp NH HS s NH g H S They are allowed to simultaneously come to equilibrium Select the correct statement 1 The ratio of amount of NH3 produced by the species is equal to the ratio of their K 2 Total pressure exerted by NH3 is greater than the total pressure exerted by CO and H S 3 Mole fraction of NH3 cannot be greater than 1 3 4 Pressure exerted by CO is twice the pressure overted by

Physical Chemistry
EquilibriumImpure copper containing Fe Au Ag as impurities is electrolytically refined A current of 140 A for 482 5 s decreased the mass of the anode by 22 26 g and increased the mass of cathode by 22 011 g Percentage of iron in impure copper is Given molar mass of Fe 55 5 g mol molar mass of Cu 63 54 g mol a 0 95 b 0 85 c 0 97 KCET 2014 d 0 90
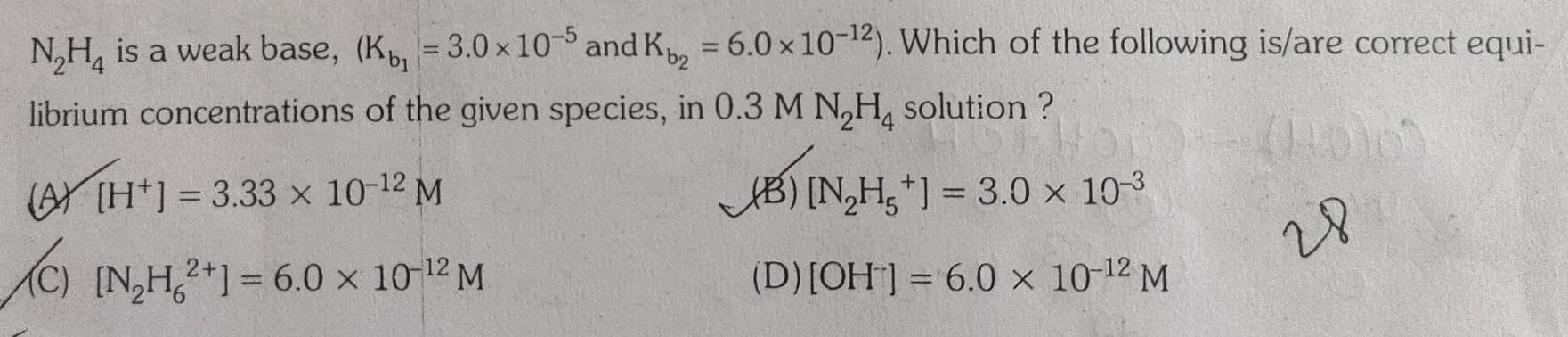
Physical Chemistry
EquilibriumN H is a weak base K 3 0 x 10 5 and K 6 0 x 10 2 Which of the following is are correct equi librium concentrations of the given species in 0 3 M N H solution 3 33 x 10 12 M B N H 3 0 10 3 C N H2 1 6 0 10 2 M D OH 6 0 x 10 2 M 28

Physical Chemistry
EquilibriumWhat is the pH of 4 x 10 3 MY OH solution assuming the first dissociation to be 100 and second dissociation to be 50 where Y represents a metal cation log 2 0 3010 log 3 0 477 A 11 778 B 11 477 C 2 523 D 2 222

Physical Chemistry
EquilibriumInstance 8 0 24 mol of each SO3 g and NO g were taken in a reaction vessel and heated to some temperature where the following equilibrium was established SO3 g NO g SO g NO g Unreacted SO3 at equilibrium were absorbed in a 250 mL 1 5 M NaOH solution 25 mL of this solution required 9 3 mL of a 1 0 M HCl solution for complete neutralization Determine K

Physical Chemistry
EquilibriumKe log RT 0 is true relationship for the log Kc following reaction 1 PCI PCI Cl 2 2502 0 250 3 3 N 3H 2NH 2 4 2 and 3 both CE0018 T a

Physical Chemistry
EquilibriumThe reaction CaCO 1 of the high temperature 3 CaO is not dissociated CaO s CO2 g goes to completion in lime kiln because 2 CaO is more stable than CaCO3 4 CO escapes continuously

Physical Chemistry
EquilibriumWhen CuSO4 reacts with Kl the oxidation number of changes by a 0 c 1 b 1 d 2

Physical Chemistry
EquilibriumFor the equilibrium NH4HS s NH3 g H S g the expression of K in terms of equilibrium pressure p is a Kp p b K p c K p 1 d Kp p

Physical Chemistry
Equilibrium30 The number of hydrogen ions in 10 ml of a solution with pH 13 is 1 1013 3 6 023 1010 2 6 023 x 108 4 6 023 1013

Physical Chemistry
EquilibriumThe following equilibrium is established when hydogen chloride is dissolved in acetic acid HCI CH COOH CI CH COOH The set that characterizes the conjugate acid base pairs is A HC1 CH COOH and CH COOH Cl B HCI CH COOH and CH3COOH Cl C CH COOH HCI and CF CH COOH D HCl Cl and CH COOH CH COOH

Physical Chemistry
EquilibriumBase samples are prepared using NaOH KOH Ba OH 2 Mg OH 2 Al OH separately or as mixture of more than one Calculate the minimum volume of 3 65 w v HCI solution required in L to ensure complete neutralisation in every case possible if total 156 gm of sample is taken

Physical Chemistry
Equilibrium85 100 mL of 0 02 M benzoic acid pK 4 20 is titrated using 0 02 M NaOH pH after 50 mL and 100 mL of NaOH have been added are a 3 50 7 b 4 2 7 c 4 2 8 1 d 4 2 8 25

Physical Chemistry
EquilibriumThe reaction cis Cr en OH 2 trans Cr en OH 2 K is first order in both directions At 25 C the equilibrium constant is 0 1 and the rate consta k is 2 10 4s In an experiment starting with the pure cis form how long would it take for half t equilibrium amount of the trans isomer to be formed In2 0 693 k

Physical Chemistry
EquilibriumFor the reaction 2CO O 2CO AH 560 kJ Two moles of CO and one mole of O are taken in a container of volume 1 L They completely form two moles of CO2 the gases deviate appreciably from ideal behaviour If the pressure in the vessel changes from 70 to 40 atm find 2006 the magnitude absolute value of AU at 500 K 1 L atm 0 1 kJ

Physical Chemistry
EquilibriumThe electrochemical cell shown below is a concentration cell MM saturated solution of a sparingly soluble salt MX M 0 001 mol dm3 M he emf of the cell depends on the difference in concentrations of M ions at the two electrodes The emf of the cell at 298 K is 0 059 V 2012 The value of AG kJ mol for the given cell is take 1F 96500 C mol B 5 7 D 11 4 A 5 7 C 11 4 The solubility product Ksp mol dm of MX at 298 K based on the information available for the given concentration cell is take 2 303 x R x 298 F 0 059 V B 4 x 10 15 D 4 x 10 A 1 x 10 15 C 1 x 10 12

Physical Chemistry
Equilibrium25ml 0 2M Ca OH is neutralised by 10ml of 1M HCl Then pH of resulting solution is 1 1 37 3 12 2 9 4 7

Physical Chemistry
EquilibriumO Calculate the solubility of AgCN in 0 4 M KCN solution forryt hounoibai bian salt 104 33 i neglecting complex formation 1 riiw noitus mol bosimninu org ii considering complex formation Given Ksp of AgCN 8 10 10 K of Ag CN 4 10 8 o votligste mootbrand SOLUBILITY CONSIDERING HYDROLYSIS

Physical Chemistry
EquilibriumThe Kp of Ag2 CrO4 AgCl AgBr and Agli a respectively 1 1 x 10 12 1 8 x 10 10 5 0 x10 12 8 3 x 10 17 Which one of the following salts w will precipitate last if AgNO3 solution is added to the solution containing equal moles of NaCl NaBr Nal and Na CrO4 1 AgCl 3 Ag CrO4 2 AgBr 4 Agl

Physical Chemistry
EquilibriumEx One mole of ammonium carbamate dissociate as shown below at 500 K NH COONH s 2NH g CO g If the pressure exerted by the released gases is 6 0 atm the value of Kp is A 7 atm B 3 atm C 32 atm bor D 8 atm

Physical Chemistry
Equilibrium54 The relation for calculating pH of a weak base is a pH pKw pKb log c 2 1 b pH pKw pKblog c W

Physical Chemistry
Equilibrium4 3 g of a saturated hydrocarbon CnH n 2 is burnt completely and all CO2 were absorbed in aqueous NaOH solution A 300 mL 2M NaOH solution was required A CnH2n 2 O2 COz H2O NaOH CO2 Na2CO3 H O Hence value of n is

Physical Chemistry
EquilibriumWhich of the following salt solution will act as a buffer squi 1 CH COONH aq 2 NH Cl aq 3 CH COONa aq 4 NaCl ac

Physical Chemistry
Equilibriumhydrolysis of the salt should be a 5 b 10 c 20 of these 77 What is the hydronium ion concentration of a 0 02 M solution of Cu2 solution of copper perchlorate The acidity constant of the following reaction is 5 10 Cu2 aq 2H O l Cu OH aq H O aq b 7x10 4 c 5x 10 4 a 1x 10 5 d 1x 10 4 for the following reaction given that the hydronium

Physical Chemistry
EquilibriumFor a reaction AB equilibrium constant is 1 66 and Kforward 0 166 hr Calculate the time in hours when concentration of B is 80 of its equilibrium concentration Given in 25 3 20

Physical Chemistry
Equilibrium53 If the rate constant of a reaction is 2 303 10 3 s 1 then the time required for the completion of 70 of the reaction is log 3 0 48 1 5 25 minute 2 12 31 minute 3 7 25 minute 4 8 67 minute

Physical Chemistry
EquilibriumWhich of the following statement is correct regarding with chemical equilibrium 1 Based on extent to which the reactions proceed to reach the equlibrium we may have negligible conc of reactants are left 2 Equlibrium is not static 3 Concentration of reactants and products becomes constant at equilibrium 4 All of these

Physical Chemistry
EquilibriumOn applying pressure to the equilibrium icewater which phenomenon will happen 2 More water will be formed 1 More ice will be formed 4 Water will evaporate 3 Equilibrium will not be disturbed

Physical Chemistry
Equilibrium143 N g 3H g 2NH 8 Kc 100M Equilibrium constant for the decomposition of 17 grams of ammonia at same temperature is 1 10 2 0 1 3 0 01 21 2 3

Physical Chemistry
EquilibriumFrom an equimolar solution of Cl and Br ions the addition of Agt will selectively precipitates Br ion K of AgCl AgBr are 1 10 10 1 10 13 respectively sp The pH of a solution which is 0 1 M in sodium acetate and 0 01 M in acetic acid pK 4 74 would be 5 74 AgCl is less soluble in aqueous sodium chloride solution than in pure water 13 1 acts as Lewis acid In the reaction I 1 Select the correct code for above A TFTT B TTTT C FTFT D FTTT Etusion Chemistry Sheet Monic Equilibrium ENC Kund

Physical Chemistry
EquilibriumA 10 M solution of AgNO3 is made 0 2 M in NH3 Given Ag NH3 2 Ag NH3 Paragraph for Question Nos 57 to 58 Select correct options A Agtleq 10 M Ag NH3 NH3 Ag NH3 C Ag NH3 2 leq 10 M K 1 5 x 104 K 4 x 10 B Ag NH3 leq 7 5 10 M D K Ag NH3 2 6 10 8

Physical Chemistry
EquilibriumWhen a gas is compressed adiabatically and reversibly the final temperature is 1 Higher than the initial temperature 2 Lower than the initial temperature 3 The same as initial temperature 4 Dependent upon the rate of compression

Physical Chemistry
Equilibriumif reaction 4KCIO 3KCIO4 KCI d KC1O dt K KCIO d KC O4 K KCIO dt d KC1 dt K KCIO the correct relation between K K and K 1 K K K K3 2 4K 3K K

Physical Chemistry
Equilibriumc 3 136 A 0 5 g sample of KH PO4 is titrated with 0 1 M NaOH The volume of base required to do this is 25 0 ml The reaction is represented as H PO OH HPO2 H O The percentage purity of KH PO4 is K 39 P 31 a 68 for 25 b 34 d 51

Physical Chemistry
Equilibrium86 In the reaction 2N2O5 4NO2 O2 initia pressure is 500 atm and rate constant K is 3 38 x 10 5 sec After 10 minutes the final pressure of N2O5 is a 490 atm c 480 atm b 250 atm d 420 atm

Physical Chemistry
Equilibrium21 What is NH in a solution that is 0 02 M NH and 0 01 M KOH K NH 1 8 105 1 3 6 x 10 5 M 2 1 8 x 105 M 3 0 9 x 10 5 M 4 7 2 x 10 5 M

Physical Chemistry
EquilibriumThe activation energies for the forward and reverse elementary reactions in the system A B are 10 303 and 8 000 kcal respectively at 500 K Assuming the pre exponential factor to be the same for both the forward and reverse steps the equilibrium constant of the reaction at 500 K is a 1 00 c 100 b 10 0 d 0 1

Physical Chemistry
Equilibrium109 A definite amount of solid NH4HS is placed in a flask already containing ammonia gas at a certain temperature and 0 1 atm pressure NH4HS decomposes to give NH3 and H S and at equilibrium total pressure in flask is 1 1 atm If the equilibrium constant K for the reaction NH4HS s NH g H S g is represented as zx 10 then find the value of z 3

Physical Chemistry
EquilibriumWhich of the following combinations would not result in the formation of a buffer solution a NH HCI b NH C1 NH c CH COOH NaCl d NaOH CH COOH

Physical Chemistry
EquilibriumWhen 36 8g N O4 g is introduced into a 1 0 litre flask at 27 C The following equilibriur 2NO g K 0 1642 atm reaction occurs N O4 g Calculate K of the equilibrium reaction What are the number of moles of N O4 and NO at equilibrium What is the total gas pressure in the flask at equilibrium What is the percent dissociation of N O

Physical Chemistry
Equilibrium4g H and 127g I are mixed heated in 10 lit closed vessel until equilibrium is reached If the equilibrium concentration of HI is 0 05 M total number of moles present at equili brium is 1 3 25 2 1 75 3 2 25 4 2 5

Physical Chemistry
Equilibrium4 100 ml of 1 M KMnO4 oxidized 100 ml of H O in acidic medium when MnO is reduced to Mn volume of same KMnO4 required to oxidize 100 ml of H O in neutral medium when MnO is reduced to MnO2 will be A 100 3 ml B 500 3 ml C 300 5 ml D 100 ml