Gaseous and liquid states Questions and Answers

Physical Chemistry
Gaseous and liquid statesA ii and iii 4 If A Go of the cell reaction D i and iv AgCl s 2H g Ag s H Clis 21 52 KJ then AG of 2AgCl s H g 2Ag s 2H 20 is A 21 52 KJ B 10 76 KJ C 43 04 KJ B ii and iv C i and iii D 43 04 KJ

Physical Chemistry
Gaseous and liquid states71 The enthalpy of neutralisation of four acids HA HB HC and HD with NaOH are 13 12 11 10 kcal mol Which salt has maximum degree of hydrolysis a 1M NaA b 1M NaB c 1M NaC 35 1M NaD ml of weak arid HA

Physical Chemistry
Gaseous and liquid states3 2 bond pairs and 3 lone pairs 4 Both 1 and 3 52 A container contains mixture of O2 and H2 If 1 mole fraction of H2 is then the ratio of volume 3 of H to O2 diffuse out initially from the container is 1 1 2 3 1 1 2 2 1 4 4 1

Physical Chemistry
Gaseous and liquid statesAt a temperature of 30 C hexane is dissolved in water at a mole fraction of 0 67 x 10 6 At this temperature the solubility of hexane in water is xsat 10 6 What is the vapor pressure of pure hexane at 30 C if the fugacity of hexane in the gas phase is equal to 0 5 bar

Physical Chemistry
Gaseous and liquid statesColumn I Process A Reversible isothermal compression of P AS sys an ideal gas system Q B Isothermal free expansion Pext 0 of Q AS an ideal gas system P C Reversible adiabatic expansion of an R AS ideal gas R D Reversible ideal gas expansion system Entropy Change 0 0 Column II 0 S Information insufficient

Physical Chemistry
Gaseous and liquid statesIs a HF Stue b HCl 2 20 c HBr EVS bases d HI s to g Of At what pressure a quantity of gas will occupy a volume of 60 mL if it occupies a volur 100 mL at a pressure of 720 mm while temperature is constant HETS 8 700mm 900mm 100

Physical Chemistry
Gaseous and liquid states0 PI Which of the following statement is correct i If A B are same gases then T2 T ii If T T then A g may be SO2 g B g may be CH4 g iii If B C are same gases then T3 T2 iv If T T T3 then A g may be CH4 B g may be SO2 C g may be SO3 g O i ii iii only Oi ii iv only O All are correct T CT3 ii iii only

Physical Chemistry
Gaseous and liquid statesAn ideal mixture of liquids A and B with 2 moles of A and 2 moles of B has a total vapour pressure of 1 atm at a certain temperature Another mixture with 1 mole of A and 3 moles of B has a vapour pressure greater than I atm But if 4 moles of C are added to the second mixture the vapour pressure comes down to 1 atm Vapour pressure of C P 0 8 atm Calculate the vapour pressures of pure A and pure B

Physical Chemistry
Gaseous and liquid states23 A mixture of two immiscible liquids nitrobenzene and water boiling at 99 C has a partial pressure of water 733 mm and of nitrobenzene 27 mm The ratio of mass of water and nitrobenzene in mixture is

Physical Chemistry
Gaseous and liquid statesA certain hydrate has the formula MgSO4 xH O A quantity of 54 2 g of the compound is heated in an oven to drive off the water If the steam generated exerts a pressure of 24 8 atm in a 2 0 L container at 120 C calculate x bacose a bre

Physical Chemistry
Gaseous and liquid states53 If m gram of a metal A displaces m2 gram of another metal B from its salt solution and if the equivalent weights are E and E respectively then the equivalent weight of A can be expressed by A E C E m xE m m x m E B E 1xE m D E m x m E

Physical Chemistry
Gaseous and liquid statesPart A 10 moles of an ideal gas undergoes reversible isothermal expansion at 300 K from 5 L to a volume of 10 L 1 Will the entropy of gas increase decrease or remain same Calculate the entropy change in gas 2 Will any work be done by on the gas If yes then calculate the work done and explain meaning of sign of work done 3 Calculate the direction and magnitude of heat flow with the surroundings 4 Will the entropy of surroundings change too remember its a reversible isothermal process If yes then calculate the change 5 What will be the change in the entropy of universe Part B Considering the expansion in part A to be irreversible answer all the five parts asked in Part A

Physical Chemistry
Gaseous and liquid states83 The solubility product of CuS Ag2S and H are 10 31 10 44 and 10 54 respectively T solubilities of these sulphides are in the orde a HgS Ag S CuS b CuS Ag2S HgS c Ag S CuS HgS d AgS HgS CuS 1997 84 The equilibrium constant for the reaction N N 3H NH ic K th

Physical Chemistry
Gaseous and liquid statesAir is compressed in a vertical cylinder by weight of piston When a weight is added to the Piston the volume of the gas decreases from 500 mL to 400 mL If another weight of same magnitude is added the volume of the gas will decreases 2nd decrease by Assume ideal behaviour A another 100 mL B less than 100 mL C more than 100 mL D Insufficient Information

Physical Chemistry
Gaseous and liquid statesCAREER IFISTE 46 The molecular weight of two molecules X and Y are 20 and 40 respectively If one litre of gaseous X at 20 C and 750 mm pressure contains N molecules the number of molecules in two litres of gaseous Y under same conditions of temperature and pressure will be 1 4N 2 2N 3 N N 4 46 3 2040 20 C 750 my feet a tay2 lit after rytter 1 4N 2 2N 3 N N 4

Physical Chemistry
Gaseous and liquid statesIIT JEE 4 An equal volume of a reducing agent is titrated sepa with 1 M KMnO in acidic neutral and alkaline me The volumes of KMnO4 required are 20 mL in 33 3 mL in neutral and 100 mL in alkaline mediun out the oxidation state of manganese in each red product Give the balanced equations for all the thr reactions Find out volume of 1 MK Cr O consu the same volume of the reducing agent is titrated in medium IIT JEE SEDIA DE n Butane is produced by the monobromination of followed by the Wurtz reaction Calculate the vol ethane at STP required to produced 55 g n butane bromination takes place with 90 yield and the reaction with 85 yield IIT JEE A mixture of H C O4 oxalic acid and NaHC O4 w 2 02 g was dissolved in water and the solution mad L 10 mL of the solution required 3 0 mL of 0 1 N

Physical Chemistry
Gaseous and liquid statesA real gas has critical temperature and critical pressure as 40 C and 10 atm respectively then liquification c gas is possible at 1 50 C and 8 atm 3 25 C and 12 atm 2 45 C and 8 atm 4 45 C and 12 atm

Physical Chemistry
Gaseous and liquid states60 litre of such a mixture The half life of 32p is 14 3 day Calculate the specific activity of a phosphorous containing specimen having 1 0 part per million 32P Atomic weight of P 31 The hydrogen atom in the ground state is excited by mann wavelength A The resulting mmute How many mill
Physical Chemistry
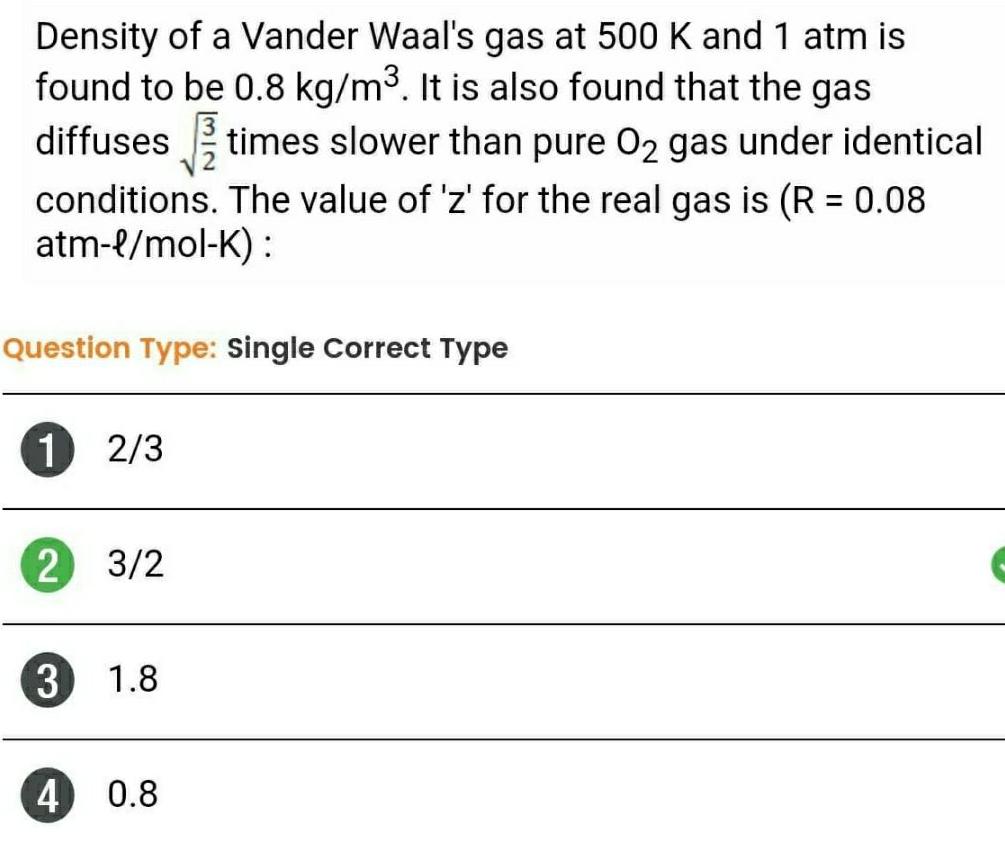
Gaseous and liquid statesDensity of a Vander Waal s gas at 500 K and 1 atm is found to be 0 8 kg m It is also found that the gas diffuses times slower than pure O gas under identical conditions The value of z for the real gas is R 0 08 atm l mol K Question Type Single Correct Type 1 2 3 2 3 2 3 4 1 8 0 8

Physical Chemistry
Gaseous and liquid statesvi A process engineer decided to use the distillation process to separate a liquid mixture consists of two components the boiling point of each compound is the same which is 80 C do you agree with this decision Justify the 2 marks reason

Physical Chemistry
Gaseous and liquid statesAt critical temperature pressure and volume The compressibility factor Z is 1 3 0 3 3 4 2 4 50 3 3 00 phos that of No O permanent ga

Physical Chemistry
Gaseous and liquid states2 If hydrogen gas is bubbled through water at 20 C then the number of millimoles of H dissolved in 2 litre of water is H exerts a partial pressure of 0 8 bar and Henry s law constant for H2 at 20 C is 69 16 K bar 1 1 29 2 12 9 3 0 129

Physical Chemistry
Gaseous and liquid statesTwo glass bulbs A and B are connected by a small tube of negligible volume having a stopcock Initially the volun of bulb A is 100 ml with some gas while bulb B is empty Upon opening the stopcock the pressure decreased by 60 The volume of bulb B must be 100 mL 150 mL 200 mL 180 mL

Physical Chemistry
Gaseous and liquid states5 In the below experiment the value of P is Imol gas A 2 mol gas B 250mm 250 mm A 250 mm C 300 mm For a reaction Partition removed at same tem perature T B 500 mm D 400 mm Pressure P

Physical Chemistry
Gaseous and liquid statesT Select the correct statement s about given graph f 8 A Expansion takes place C Process is not isobaric A I C B Process is not isothermal D Process is not isochoric B D

Physical Chemistry
Gaseous and liquid statesa P P c P P b P P d All of these 22 Two flasks A and B of 500 mL each are respectively filled with O and SO2 at 300 K and 1 at pressure The flasks will contain a the same number of atoms b the same number of molecules c more number of moles of molecules in flask A as compared to flask B d the same amount of gases ylem d d ALEBO C

Physical Chemistry
Gaseous and liquid states40 The P of real gases is less than the P of an ideal gas because of A Increase in number of collisions B Finite size of molecule C Increase in KE of molecules D Intermolecular forces D coate 148 Whic spon cell A B B F

Physical Chemistry
Gaseous and liquid statesFor an ideal gas sample fixed amount taken in fixed volume container collision frequency z mean free path X 1 Both Z A changes with change in T ii Z changes with change in T iii A changes with change in T iv Both are independent of temperature T v Heavier gas more molecular weight exibits higher Z compared to lighter gas if collision diameter of heavier gas is mor vi Heavier gas exibits more A compared to lighter gas vii Molecular weight of gas does not have any effect of value of A Identify number of correct statements from above

Physical Chemistry
Gaseous and liquid statesA mercury column of length 10 cm is in the middle of a horizontal tube of length 1 m closed at both ends containing same amount of a gas in both columns If the tube is placed vertically the mercury column will shift through a distance of 15 cm from its initial position Find the final pressure of gas in upper column in cm of Hg when the tube is placed vertically

Physical Chemistry
Gaseous and liquid states26 The mass of molecule AB is four times the mass of molecule AC The rms velocity of AB is three times the rms velocity of AC The ratio of pressure exerted by AB AC in two containers of equal volume and at the same temperature is 2 12 1 4 36 1 1 1 12 3 1 36

Physical Chemistry
Gaseous and liquid statesDraw a PC pH diagram for a diprotic acid with a Ka1 10 5 M K 2 109 and CTA 10 3 M At what pH is the concentration of A 2 is 10x greater than the concentration of hydroxide ion HO At what pH is the concentration of HA is 1 000x greater than the concentration of hydroxide ion HO

Physical Chemistry
Gaseous and liquid states2 In the test tubes A and B shown here yeast was kept in sugar solution Which products of respiration would you expect in test tubes A and B i Acrobic Anaerobic Oil film F Sugar solution Yeast 7 Tes

Physical Chemistry
Gaseous and liquid states144 The rates of diffusion of SO3 CO PC13 and SO are in the following order A PCI 3 SO3 SO CO B CO SO PCI 3 SO3 C SO SO3 PCI3 CO D CO SO SO3 PC 3

Physical Chemistry
Gaseous and liquid statesThe diffusion coefficient of an ideal gas is proportional to the mean free path and mean speed The absolute temperature is made 9 times while the pressure is increased 3 times As a result the diffusion coefficient becomes x times The value of x is

Physical Chemistry
Gaseous and liquid statesIn an experiment similar to the one you will be conducting this week the vapor of a 0 475g of an organic alcohol filled up a 250 mL Erlenmeyer flask at 97 degrees C and 730 mm Hg What is the molar mass of the alcohol

Physical Chemistry
Gaseous and liquid statesAt 0 C when the density of a certain oxide of a gas at 3 bar is same as that of dioxygen at 5 bar the molecular mass of the oxide is Options 53 3 45 5 19 2 20 7 Solution

Physical Chemistry
Gaseous and liquid statesVander waal s gas equation may be expressed as B C Z 1 V m V m Where V molar valume of gas If B 0 105 L mol and C 4 x 10 4L mol 2 at 127 value of Vander waal constant a in atm L mol 2 is R 0 08 L atm K mol

Physical Chemistry
Gaseous and liquid states80 ml of O takes 2 minute to pass through the hole what volume of SO will pass through hole in 3 min S 32 O 16 2 120 2 3 120 2 12 S 4 12 2 Vander waal constant a accounts for

Physical Chemistry
Gaseous and liquid states0 In a closed flask of 5 litres 1 0 g of H is heated from 300 to 600 K Which statement is not correct a Pressure of the gas increases b The rate of collision increases c The number of moles of gas increases d The energy of gaseous molecules increases 1991

Physical Chemistry
Gaseous and liquid states7 An ideal gas can t be liquefied because a its critical temperature is always above 0 b its molecules are relatively smaller in size c it solidifies before becoming a liquid d forces operative between its molecules are negligible 1992

Physical Chemistry
Gaseous and liquid states33 When is deviation more in the behaviour of a gas from the ideal gas equation PV nRT a At high temperature and low pressure b At low temperature and high pressure c At high temperature and high pressure d At low temperature and low pressure 1993 A closed flack contains water in all its three

Physical Chemistry
Gaseous and liquid statesincreases 1991 At constant temperature in a given mass of an ideal gas a the ratio of pressure and volume always remains constant b volume always remains constant c pressure always remains constant d the product of pressure and volume always remains constant 1991 The root mean square velocity at STP for the 46 II P mass respe is gi a 47 Press a giv every by de

Physical Chemistry
Gaseous and liquid states4 A closed flask contains water in all its three states solid liquid and vapour at 0 C In this situation the average kinetic energy of water molecules will be a the greatest in all the three states b the greatest in vapour state c the greatest in the liquid state d the greatest in the solid state 1992

Physical Chemistry
Gaseous and liquid states164 At low pressures For 1 mole the Vander Waal s equation is written as a P The compressibility factor is then equal to 1 RTV A C 1 V RT a RTV a RTV B D 1 RTV a

Physical Chemistry
Gaseous and liquid statesZero darkened Negative Marks 1 In all 1 Combustion of 50 mL methane is carried with 150 ml of air containing 21 of oxygen What will be the total volume of gaseous mixture after reaction CH 20 CO g 2H O g 2 8 2 110 mL 4 144 5 mL 58 1 200 ml 3 113 mL The mass of glucose that would be dissolved of water in order to produce the same pressure as is produced by the same quantity

Physical Chemistry
Gaseous and liquid statesme spontaneou 1 The solubility of NaCl in water at 25 C is about 6 moles per litre Suppose you add 1 mole of NaCl to a litre o water For the reaction NaCl H O Salt solution a AG 0 AS 0 c AG 0 AS 0 b AG 0 AS 0 d AG 0 AS 0

Physical Chemistry
Gaseous and liquid statesThe compression factor Z of certain a gas is equal to 0 83 when the temperature is 315 K and the pressure is 23 atm How much volume 5 7 mmol of the will occupy under these conditions

Physical Chemistry
Gaseous and liquid states8 Two gases A and B having the same volume diffuse through a porous partition in 20 and 10 seconds respectively The molecular mass of A is 49 u Molecular mass of B will be a 50 00 u c 6 50 u b 12 25 u d 25 00 u

Physical Chemistry
Gaseous and liquid statesAn open container of volume V contains air at temperature 300 K The container is heated to such a temperature so that amount of gas coming out is 2 3 of the amount of gas initially present in the container Find the temperature to which the container should be heated a 300 K c 900 K b 600 K d 1200 K

Physical Chemistry
Gaseous and liquid statesmolecular mass of B is M then molecular mass of A is a M b 4 M C M d 2 M