Gaseous and liquid states Questions and Answers

Physical Chemistry
Gaseous and liquid statesPV value decreases with increase in P at constan temperature when a there is no attractive or repulsive forces between molecules b attractive and repulsive forces between molecule are equal c attractive forces between predominant d repulsive forces between predominant molecules ar molecules ar J K CET

Physical Chemistry
Gaseous and liquid statesAssuming complete ionization same m which of the following compounds will require the least amount of acidified KMnO4 for complete oxidation Re AIPMT 2015 1 FeC 04 3 FeSO4 2 Fe NO 2 4 FeSO3 invo

Physical Chemistry
Gaseous and liquid statesThe Vrms of a missed particle having a mass of 10 12g at 27 C if value of Boltzmann constant k is 1 31 x 10 16 m sec Find the value of 2x Consider the nearest integer ergs per degree per molecule is x

Physical Chemistry
Gaseous and liquid statesIn the reaction 6CH 3H g aq aq 2AL 6HCl 2A1 1 6L HC1 is consumed for every 3L H produced aq 2 33 6 L H is produced regardless temperature and pressure for every moles that reacts 3 67 2 L Hat STP is produced for every mole of Al that reacts 1 2 LH at STP is produced for every mole of HCI consumed COM ulphuric acid solution that is 29 H SO molar mass 98 g ma

Physical Chemistry
Gaseous and liquid statesThe normal boiling point of a liquid X is 500 K Which of the following statement s is are incorrect for the following process X liquid X gas At 500 K and 1 atm pressure AG 0 At 500 K and 1 5 atm pressure AG ve At 500 K and 0 2 atm pressure AG ve At 520 K and 1 atm pressure AG ve

Physical Chemistry
Gaseous and liquid states23 4 If we plot volume of a certain mass of a gas against temperature at constant pressure we get a straight line interesting on the negative side at 273 C which explains about absolute zero This graph is known as X 273 C 1 Isochore 2 Isotherm 3 Isotone 4 Isobar Which of the following is not an allylic holide 0 T C

Physical Chemistry
Gaseous and liquid states20 Correct order of freezing point of given solution I 0 1 M glucose 1 IV II 0 2 M urea 2 IV III 0 1 M 3 IV Nacoun IV 0 05 M CaCl 4 IV Services Limite

Physical Chemistry
Gaseous and liquid statesWhich gas shows real behaviour 16 g of dioxygen at STP occupies 11 2 L One gram dihydrogen in 0 5 L flask exerts pressure of 24 63 atm at 30 One mole of ammonia at 300 K and 1 atm pressure occupies 22 4 L

Physical Chemistry
Gaseous and liquid statesAt 570 mm Hg and 298 K a gas sample has a volume of 2270 mL What is the final pressure in mm Hg at a volume of 1250 mL and a temperature of 448 K if the number of moles are constant O 700 mm Hg O 690 mm Hg O 210 mmHg 1560 mm Hg O 470 mm Hg

Physical Chemistry
Gaseous and liquid statesA gaseous mixture was passed at the rate of 2 5L min through a solution of NaOH for a total of 1 hour The SO in the mixture was retained as sulphite ion SO g 2OH SO g H O l After acidification with HCl the sulphite was titrated with 5ml of 0 003M KIO 10 2H SO 2Cl250 IC 2H H O Calculate concentration of SO2 in ppm if density of gaseous mixture is 1 6 gm L

Physical Chemistry
Gaseous and liquid statesQuestion Type Single Correct Type 1 2 3 a 3 2 P P P P V 0 2b 0 2RT a v 0 04V V b 0 02RT 0 04a 2 V V 0 2b 0 2RT 0 2a

Physical Chemistry
Gaseous and liquid states40 The equilibrium of which of the following reactions will not be disturbed by the addition of inert gas at constant volume AH g 1 g 2HI g C N O4 g 2NO g B PC1 g PCl g Cl g D N g O g 2NO g

Physical Chemistry
Gaseous and liquid statesIdentify the incorrect statement among the following a Van der Waal s constant a is a measure of intermolecular force of attraction between the molecule b Intermolecular force of attraction increases with the increase in pressure c Van der Waal s constant a becomes negligible at high pressure d None of these

Physical Chemistry
Gaseous and liquid statesQuestion 4 3 marks Three ideal gases are mixed in a 3 5 L vessel The vessel is connected by a valve to a 5 0 L chamber This second chamber contains no gas at all it is a vacuum The three gases in the 3 5 L vessel helium neon and argon have the following partial pressures 17 0 kPa 22 0 kPa and 32 0 kPa What is the total pressure in the two chamber system after the valve is opened

Physical Chemistry
Gaseous and liquid statesWhen water is heated from 0 C to 10 C its volume 1 2 3 4 Correct Answer 4 Your Answer 1 Increase Decrease Does not change First decreases and then increases

Physical Chemistry
Gaseous and liquid statesOne mole of ice is converted into the water at 273 K The entropies of H O s and H O 1 are 38 20 and 60 01 J mol K respectively The enthalpy change for the conversion is 1 59 54 J mol 2 5954 J mol 3 595 4 J mol 4 320 6 J mol

Physical Chemistry
Gaseous and liquid statesThe entropy change in the fusion of one mole of a solid melting at 27 C the latent heat of fusion is 2930 J mol 2 is 3 1 9 77 JK mol 2 10 73 JK mol 3 2930 JK mol 4 108 5 JK mol 4 7 F

Physical Chemistry
Gaseous and liquid stateswhich of the following curve is are correct A B Free energy A g B g K 1 G Pure A Free energy Pure A Free C energy D 1 0 Pure A X 0 5 X 0 5 A g B g K 1 X 0 5 x 0 5 A g B g K 1 Pure B G T T T T A Ideal gas 200 400 600 p 101 325 kPa X 0 5 Pure B X 0 5 OR Pure B

Physical Chemistry
Gaseous and liquid states1 2 3 4 ARJUNA JEE Mole Concept Continued Based on the following consecutive reactions 2KCIO 2KCI 30 4A1 30 2Al O3 2 moles of KCIO of 50 purity on strong heating and then with excess of Al Al2O3 formed is A 2 mol C 3 mol In the following set of reactions CaCO CaO CO 75 yield CO C2CO 50 yield CO formed at STP from 10 g of CaCO is A 2 24 L B 11 2 L C 1 12 L D 1 68 L II III B 1 mol D 1 5 mol NH is formed in the following step Ca 2C CaC 50 yield L CaC N CaCN C 100 yield CaCN 3H 0 2NH3 CaCO 50 yield 2 moles of NH are formed if we take A 1 mol Ca B 2 mol Ca C 3 mol Ca D 4 mol Ca What weights of P O and P O will be produced by the combustion of 31 g of P in 32 g of oxygen leaving no Pa and O A 2 75 g 219 5 g B 27 5 g 35 5 g C 55 g 71 g D 17 5 g 190 5 g 5 How many moles of P4O6 and P4O10 will be produced by the combustion of 12 4 gm of phosphorus in 12 8 gm of Oz leaving no Pa or 0 Atomic wt P 31 A 0 11 mol and 0 3 mol B 0 15 mol and 0 25 mol C 0 05 mol each D 0 1 mol each 6 If we start from 500 g of CaCO3 and treated with series of reagents to give C H o find mass of CsH formed if molar mass of CaCO is 100g and CsHio is 58 g B 116 g A 58 g C 168 g D 224 g 7 For sequential reaction A B C 2B C 2D If yield of i and ii reactions are 90 and 80 respectively then the overall yield is expected to be A 90 C 72 A 42 gm C 14 3 gm i ii B 80 D 10 8 NX is produced by the following step of reactions M X MX 3MX2 X M Xs MiXs N CO3 NX CO M 0 How much M metal is consumed to produces 206 gm of NX Take at Wt of M 56 N 23 X 80 B C Z F A 3 moles C 5 moles DPP 07 B 56 gm D 7 4 gm 5 moles of A 6 moles of Z are mixed with sufficient amount of C to finally produce F Then find the maximum moles of F which can be produced Assuming that the product formed can also be reused Reactions are A 22 1 B 4 5 moles D 6 moles 10 Human lungs can absorb 8 gm O per hour by respiration If all oxygen atoms are converted to carbohydrates C6H12O6 how long will it take to produce 180 gm C6H12O6 A 8 hours B 12 hours C 10 hours D 6 hours

Physical Chemistry
Gaseous and liquid states3 If E Au Au 0 volt then E is 1 69 volt and E Au Au 3 1 0 19 V 3 1 255 V will be 3 Au Au 2 1 255 V 4 2 51 V is 1 40 83

Physical Chemistry
Gaseous and liquid statesa 1 8 c 1 16 4 The cell reaction for the given cell is spontane Pt H H M H M Pt H A a P P c P P b P P d Pj 1 atm of e ampere was passed

Physical Chemistry
Gaseous and liquid statesto 300 K a 2 g b 3g c 4 g d 1 g gases neon 13 A vessel contains two non reactive monatomic and oxygen diatomic The ratio of their partial pressures is 3 2 The ratio of number of molecules is b c d 3 14 A cylinder contains 10 kg of gas at a pressure of 312 E

Physical Chemistry
Gaseous and liquid statesQuestion No 4 If enthalpy of combustion of methane is x kJ mol then heat evolved by the combustion of 8 g CH4 is x2 C O x kJ O 8xkJ O 16 x kJ

Physical Chemistry
Gaseous and liquid states1 A 5 5 L balloon is completely filled with air indoors at a temperature of 21 0 C The balloon is taken out on a cold winter day If the final volume of the balloon is 4 5 L what is the final temperature in Celsius 3 marks

Physical Chemistry
Gaseous and liquid statesWhich of the following is incorrect regarding Gibbs energy Question Type Single Correct Type 1 It is a state function 2 It is a macroscopic property 3 Change in Gibbs energy decide spontaneity of a process A It is intensive property

Physical Chemistry
Gaseous and liquid statesa c 439 3 Considering thermodynamic entropy S as parameter the criterion for the spontaneity of any process is a ASsystem AS surroundings 0 b AS system AS surroundings 0 c AS system 0 only d AS surroundings 0 only 200 the expansion

Physical Chemistry
Gaseous and liquid statesIf 4g of oxygen diffuse through a very narrow hole how much hydrogen would have diffused under identical conditions 1 16 g 2 1 g 3 1 4 g 4 64 g 4 rough the

Physical Chemistry
Gaseous and liquid statesN g reacts with H g in either of the following ways depending upon supply of H g N g H g N H 1 N g 2H g N H g If 5LN g and 3 LH g are taken initially at same temperature and pressure Calculate the concentration in volume after the reaction in L

Physical Chemistry
Gaseous and liquid statesSelect the incorrect statement For solid liquid equilibrium there is only one temperature at 1 atm at which both the phases can coexist Vapour pressure depends on temperature For dissolution of solids in liquids the solubility is constant at a given temperature For dissolution of gases in liquids the concentration of a gas in liquid is independent to the pressure of the gas over the liquid

Physical Chemistry
Gaseous and liquid statesHow many kilojoules of energy is required to heat 54 0 g H 0 3 00 moles from a liquid at 19 0 C to a gas at 124 0 C The following physical data may be useful AHvap 40 7 kJ mol Cliq 4 184 J g C Cgas 2 01 J g C Csol 2 09 J g C Tmelting 0 C Tboiling 100 C

Physical Chemistry
Gaseous and liquid statesIf a gas expands at constant temperature it indicates that a kinetic energy of molecules remains the same b number of the molecules of gas increases c kinetic energy of molecules decreases d pressure of the gas increases 2008

Physical Chemistry
Gaseous and liquid statesWhich is an incorrect statement 1 Diamond is unaffected by conc acids but graphite reacts with hot conc HNO3 forming mellit acid C6 COOH 2 CO is toxic because it forms a complex with hemoglobin in the blood 3 CO carbon suboxide is a foul smelling gas 4 COCI is called tear gas Which test is used to distinguish aldehydes from Ketones

Physical Chemistry
Gaseous and liquid statesSolveLancer Test The rate of diffusion of two gases X Y are in the ratio of 1 8 If the ratio of their mass is present in the mixture is 8 9 Calculate the ratio of their moles SolveLancer Test 3 O a O b O C 11914NITAIW a b 6 d A

Physical Chemistry
Gaseous and liquid states59 180 Consider the following reaction Red hot H BO3 B A and B respectively are B H6 B O3 B O3 HBO HBO2 B O3 Mark for Review B O3 B H6

Physical Chemistry
Gaseous and liquid states9 gmp Consider the following liquid vapour equilibrium Liquid Vapour Which of the following relations is correct aup 1 3 dinP AH dT dinP dT 10 If the C RT AH RT 2 T 4 equilibrium duloa riliw a lODA ONO dinG AH dinP dT NEET 2016 RT2Nq AH T 1 constant 8 viilidulos ent 1 1

Physical Chemistry
Gaseous and liquid statesEXTRACTION OF ALUMINIUM 123 Bauxite ore can be represented as AIO OH 2 the value of x ranges between 1 0 to 1 3 0 to 1 5 4 3 vide impurities 2 1 to 2 4 0 to 1 3 associated with bauxit

Physical Chemistry
Gaseous and liquid statesAt 300 K and 1 atm 15 mL of a gase hydrocarbon requires 375 mL air contai 20 O by volume for complete combust After combustion the gases occupy 330 Assuming that the water formed is in li form and the volumes were measured a same temperature and pressure the form of the hydrocarbon is JEE Main 2016 Off a C3H8 b C4H8 c C4H10 d C3H

Physical Chemistry
Gaseous and liquid states1 In this question you will demonstrate your ability to convert between pressure units use correct significant digits and also to use the gas laws There is a fixed amount of gas in a flexible container Calculate the missing quantity for your line of the table 6 marks App Name Imran Mohamed Olivia Aiden Arlena Jenni Anthony G Kaarunya Nika Seraph Lavigne Leon Lily Linger Anthony M Farhan Madeline Parto Stewart Ujin Aaron Benson Adriana Shiv Sam William Chandler P Torr 150 0 160 0 270 0 280 0 290 0 300 0 310 0 320 0 330 0 340 0 Akkshayaa 350 0 360 0 370 0 380 0 390 0 400 0 410 0 Doris Corwin 170 0 180 0 190 0 200 0 210 0 220 0 230 0 240 0 250 0 260 0 420 0 240 0 180 0 V L 32 0 410 0 0 45 35 5 0 0125 15 0 23 0 4 25 8 5 75 0 22 0 1 65 500 0 2 25 10 5 125 2 80 12 6 225 3 50 14 8 325 4 90 15 5 410 0 7 5 47 5 55 0 75 0 35 5 T C 30 0 25 0 20 0 15 0 10 0 5 0 5 0 10 0 15 0 20 0 25 0 30 0 35 0 40 0 45 0 50 0 55 0 60 0 65 0 70 0 75 0 80 0 85 0 90 0 95 0 100 0 105 0 110 0 20 0 15 0 P2 kPa 50 5 150 0 350 0 200 0 95 0 85 0 110 0 94 0 225 101 155 87 0 120 0 75 0 128 145 78 0 95 0 V L 15 0 0 75 12 5 35 0 49 0 10 0 25 0 0 95 200 0 5 50 75 0 6 0 108 11 4 111 5 6 100 0 105 75 0 25 0 12 5 T C 150 0 25 0 25 5 50 0 100 0 65 0 38 0 40 0 85 0 90 0 160 0 98 0 115 105 50 0 212 45 0 155 0 35 0 38 0 25 5

Physical Chemistry
Gaseous and liquid states3 A mixture weighing 228 g contain CaCl and NaCl If this mixture is dissolved in 10 kg of water and form ideal solution that boil at 100 364 C The mol of NaCl in mixture is K of water 0 52 K mol kg 1 33 3 3 50 2 66 67 4 75

Physical Chemistry
Gaseous and liquid statesKm KT 22 One half mole each of nitrogen oxygen and carbon dioxide are mixed in enclosure of volume 5 litres and temperature 27 C The pressure exerted by mixture is R 8 31 mol K a 7 48 x 105 Nm b 5x105 Nm c 6 x 105 Nm d 3 x 10 Nm 23 From a certain apparatus the diffusion rate of
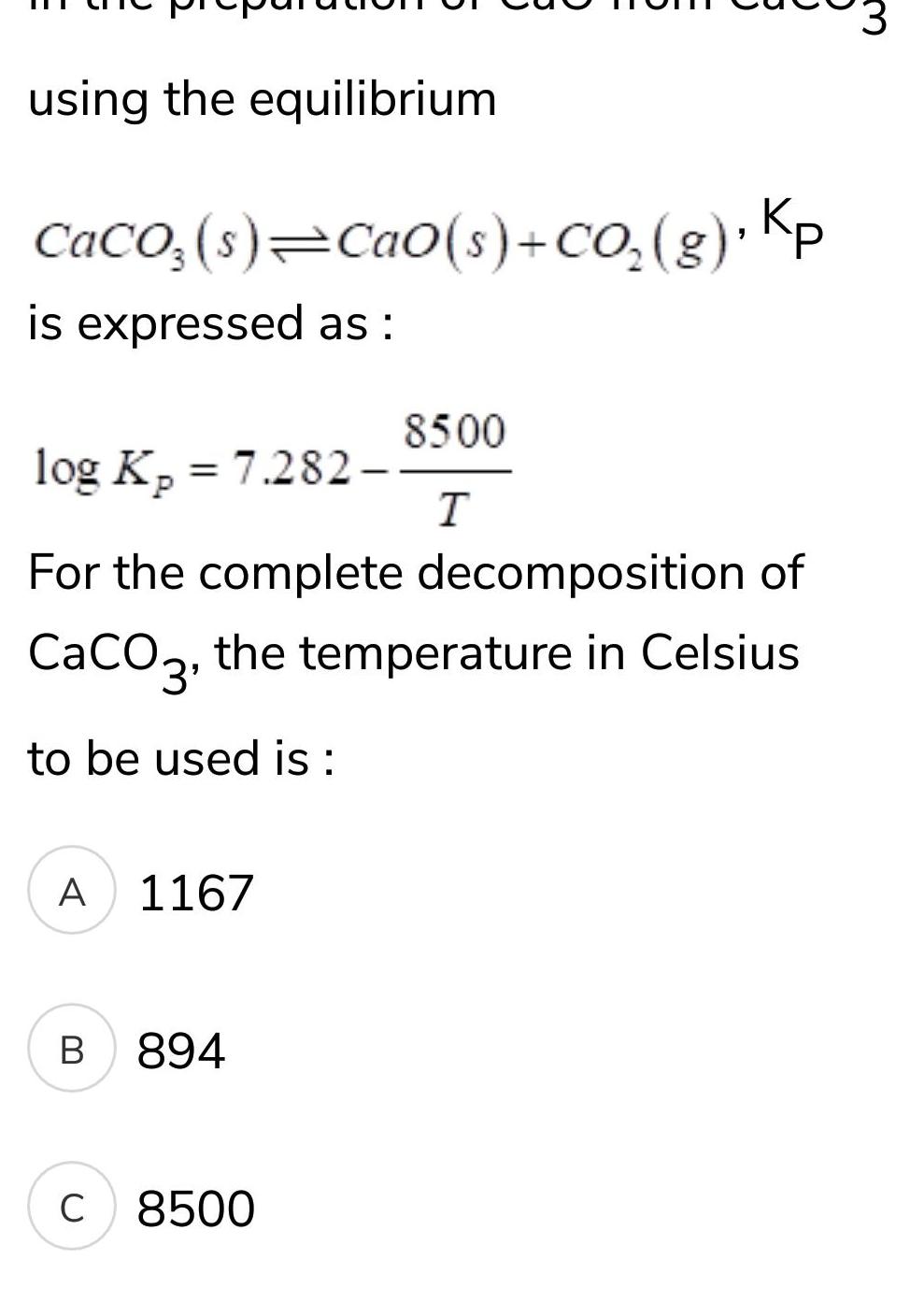
Physical Chemistry
Gaseous and liquid statesusing the equilibrium CaCO3 s Ca0 s CO g Kp is expressed as log K 7 282 Kp For the complete decomposition of CaCO3 the temperature in Celsius to be used is A 1167 B 894 8500 T C 8500

Physical Chemistry
Gaseous and liquid statesFor the equilibrium LiCl 3NH3 s LiCl NH 3 s 2NH3 g 2 K 9 atm at 37 C A 5 litre vessel contains 0 1 mole of LiCl NH 3 How many moles of N should be added to the flask at this temperature to derive the backward reaction completion Use R 0 082 atm L mol K a 0 2 b 0 59 c 0 69 d 0 79

Physical Chemistry
Gaseous and liquid statesIf 1 mole of a non ideal gas initially at 325 K and occupying 5 0 L is allowed to expand isobarically to twice the original volume what is the work done by the gas Note 1 355 L atm mol a b 3 201 x 10 2 L mol R 0 0821 1 L atm Lxatm molex K 101 325 J Provide your answer in kilojoules using only numbers Ignore the sign on the value

Physical Chemistry
Gaseous and liquid states5 The total pressure of a mixture of hydrogen helium and argon is 99 3 kPa If the partial pressure of helium is 42 7 kPa and the partial pressure of argon is 54 7 kPa what is the partial pressure of hydrogen

Physical Chemistry
Gaseous and liquid statesQ 28 A sample of Nitrogen gas was 1 placed in a flexible 9 litre container at 300k at a pressure of 1 5 atm The gas was compressed to a volume of 3 0L and heat was added until the temperature reached 600k What is the new pressure inside the container

Physical Chemistry
Gaseous and liquid statesWhen air expands adiabatically without gaining or losing heat its pressure P and volume V are related by the equation PV1 4 C where C is a constant Suppose that at a certain instant the volume is 420 cubic centimeters and the pressure is 77 kPa and is decreasing at a rate of 13 kPa minute At what rate in cubic centimeters per minute is the volume increasing at this instant cm min Pa stands for Pascal it is equivalent to one Newton meter squared kPa is a kiloPascal or 1000 Pascals

Physical Chemistry
Gaseous and liquid states136 The pressure at the bottom of a tank of water is 3P where P is atmospheric pressure If the water is drawn out until the level of water is lowered by one fifth then the pressure at the bottom of the tank is 1 2P 2 3 8P 5 4 4P 5 13P 5 137 4 140 M M The d y 5 x tis in s 1 5000 2 2 m s 3 0 5 m 4 300

Physical Chemistry
Gaseous and liquid statesDEVIATION OF REAL GASES FROM IDEAL GAS BEHAVIOUR At very low pressure and high temperature real gases behave as ideal gas De from ideal gas behaviour is mathematically denoted by Z named as compressibility factor low pressure Z V real V ideal VobsP nRT Pre Medical C P Vm obs RT

Physical Chemistry
Gaseous and liquid statesAs the temperature is raised from 20 C to 40 C the average kinetic energy of neon atoms changes by a factor of which of the following 1 1 2 2 2 3 Which one of the following aqueous solutions will exhibit highest elevation in boiling point 1 0 05 M glucose non ionisable 313 293 313 293

Physical Chemistry
Gaseous and liquid statesWhich of the following is not true as per the kinetic theory of gases V container Vvacant space F van der Wall F dipole dipole 0 KE average Constant at cont temperature Position and time relationship can not be given for a given particle of gas