General Questions and Answers

Physical Chemistry
GeneralSelect all that apply.
Which of the following bases are strong enough to deprotonate CH3CH₂CH₂C=CH (pK₂ = 25), so that
equilibrium favors the products?
CH3ONa
NaC=N
CH3Li
NaCH₂(CO)N(CH3)2
CF3COONa
CH3NHNa

Physical Chemistry
GeneralComplete and balance the equations for the given single displacement reactions. Write the reaction in molecular form. Phases are optional.
If you need to clear your work and reset the equation, click the CLR button.

Physical Chemistry
GeneralPhosphorus reacts with oxygen to form diphosphorus pentoxide, P₂O5.
4 P(s) + 50₂(g) - 2 P₂O5 (s)
How many grams of P₂O5 are formed when 3.37 g of phosphorus reacts with excess oxygen? Show the unit analysis used for
the calculation by placing the correct components into the unit-factor slots.

Physical Chemistry
GeneralA student has a tube containing solid ammonium carbonate. He opens the tube and wafts the gases towards his nose. A strong odor is detected.
Write the balanced chemical equation for this reaction involving solid ammonium carbonate. Include states of matter. One of the
products is ammonium hydrogen carbonate.
chemical equation:
This is an example of a reaction.

Physical Chemistry
GeneralA chemist titrates 110.0 mL of a 0.1696 M hydrobromic acid (HBr) solution with 0.3198 M NaOH solution at 25 °C. Calculate the pH at equivalence.
Round your answer to 2 decimal places.
Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of NaOH solution added.

Physical Chemistry
GeneralA 1.912-g sample of calcium chloride is decomposed into its constituent elements and found to contain 0.690 g Ca and 1.222 g Cl. Calculate the mass percent composition of Ca and Cl in calcium chloride.

Physical Chemistry
GeneralA 14.571 g sample of CaCl, was added to 28.032 g of K₂CO, and mixed in water. A 3.531 g yield of CaCO, was obtained.
What is the limiting reagent?
K₂CO3
CaCl₂
CaCO3

Physical Chemistry
GeneralIt is not unusual for a simple unit conversion to require two steps. Consider Michelle's problem. The length of the dowel used to make the perch of the birdhouse is 1.016 dm. How many centimeters is this? You have definitions relating decimeters to meters and centimeters to meters, but none relating decimeters to centimeters. As a result, you must convert 1.016 dm to meters and then to centimeters as follows:
1) 35 mg
2) 0.14 dL
3) 832.5 nm =
4) 0.0003 L

Physical Chemistry
GeneralA compound containing carbon and hydrogen has a molar mass of 112.21 g/mol and an empirical formula of CH₂.
Part A
Determine its molecular formula
Express your answer as a chemical formula.

Physical Chemistry
GeneralWrite the balanced chemical equation for the reaction in which solid calcium is added to warm water. Include the states of matter.
This reaction is an example of a reaction.
double-replacement
single-replacement
combination
combustion
decomposition


Physical Chemistry
GeneralIf 1.20 moles of Copper react with Mercury I Nitrate,
how many moles of mercury form?
B.) If 4.808 x 1025 atoms of Hg were produced, find the
Kj of heat absorbed in the reaction.

Physical Chemistry
GeneralWhich of the following concepts can be used to explain the difference in acidity between acetic acid (CH3COOH) and ethanol (CH3CH₂OH)?
Size
Resonance
Hybridization
Saved
Electronegativity

Physical Chemistry
GeneralConsider the combustion of methane given below. If 32 g of methane are burned,
how much oxygen is consumed as well? (MW CH4 = 16.04 g/mol; MW O₂ = 32.00 g/mol)
CH4 (g) + 2O2 (g)⇒CO2(g) + 2 H₂O (/)
32 g
128 g
64 g
1.0 g
4.0 g

Physical Chemistry
GeneralA sugar crystal contains approximately 2.1x10¹7 sucrose (C12 H22011) molecules.
What is its mass in milligrams?

Physical Chemistry
GeneralMaalox and Mylanta are both antacids that contain aluminum hydroxide as their active ingredient. Write the balanced equation for the neutralization of hydrochloric acid with aluminum hydroxide, Al(OH)3. Include physical states.

Physical Chemistry
GeneralSamples of several compounds are decomposed, and the masses of their constituent elements are measured. Calculate the empirical formula for each compound.
9.447 g Ca, 7.557 g S, 15.107 g O
Express your answer as a chemical formula.

Physical Chemistry
GeneralWhat is the net ionic equation for
the reaction below?
MnS(s) + H₂SO4 (aq) → MnSO4 (aq) + H₂S(g)
MnS(s) + 2H+(aq) → Mn²+ (aq) + H₂S(g)
Mn(s) + SO42- (aq) → MnSO4 (aq)
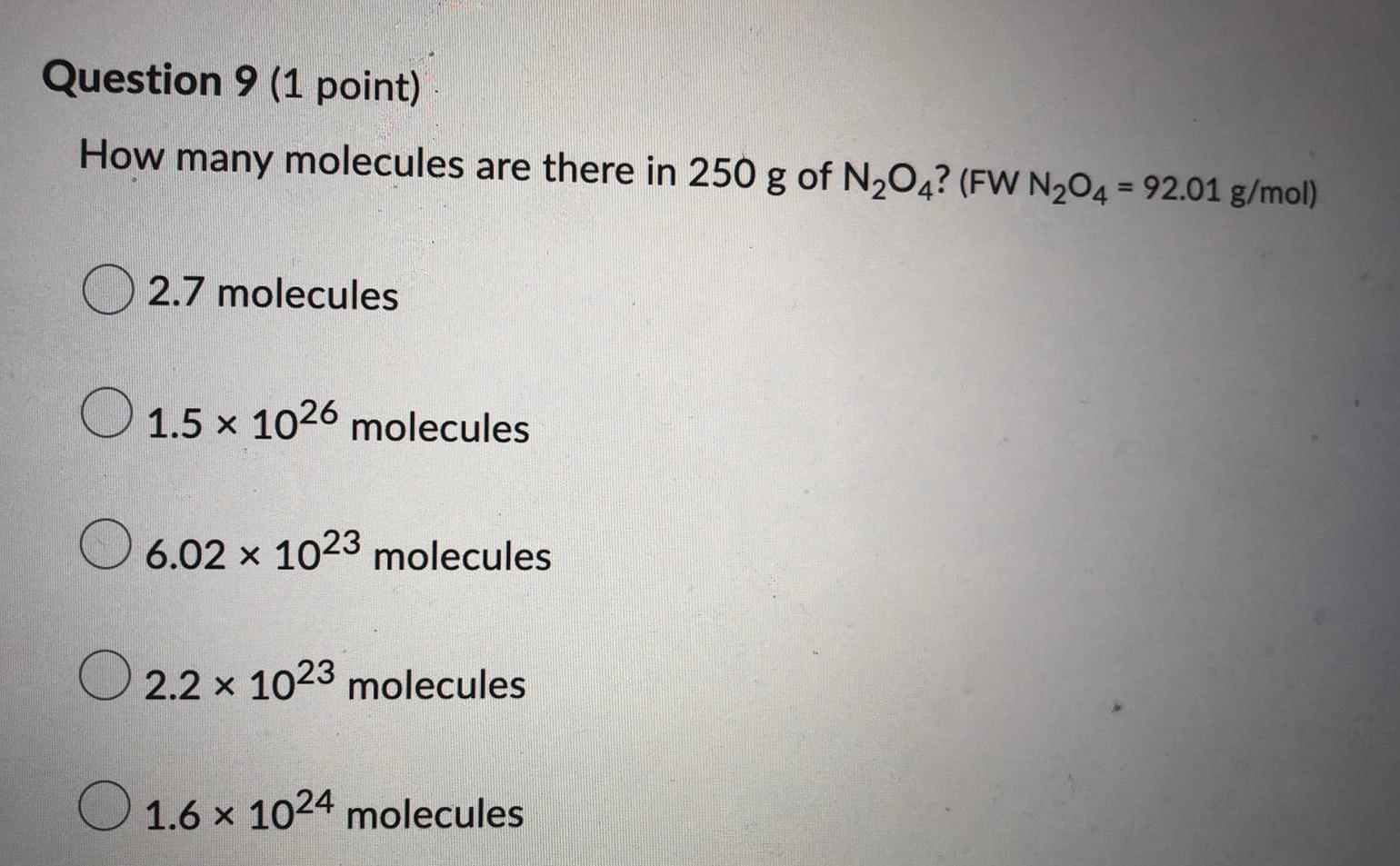
Physical Chemistry
GeneralHow many molecules are there in 250 g of N₂O4? (FW N₂O4 = 92.01 g/mol)
2.7 molecules
1.5 × 1026
1.5 x1026 molecules
6.02 x 1023 molecules
2.2 x 1023 molecules
1.6 x 1024 molecules

Physical Chemistry
GeneralIn the reaction below, if 21.31 g of H2(g) reacts completely, then how many moles of
ammonia (NH3) would form? (MW N₂ = 28.01 g/mol; MW H₂ - 2.016 g/mol, MW NH₂ =
17.03 g/mol)
N2(g) + 3 H2(g)⇒2 NH3(g)

Physical Chemistry
GeneralThe reaction below is run with 3.90 mol of Sg to completion. After running the
experiment, only 8.47 mol of disulfur dichloride were collected. What was the
percent yield of this reaction? (MW Sg = 256.48 g/mol; MW S₂Cl2 = 135.02 g/mol)
S8 (s) + 4 Cl2 (g)-> 4 S₂Cl2 (1)

Physical Chemistry
GeneralIn the reaction below, if 10 mol of N2O5 (g) decompose, then how many moles of
oxygen would form? (MW N2O5 = 108.01 g/mol; MW O₂ = 32.00 g/mol)
2 N₂O5 (g)⇒4 NO2 (g) + O2(g)
5 mol
20 mol
1 mol
10 mol

Physical Chemistry
GeneralConsider the following precipitation reaction:
_K₂SO3(aq) + _AgNO3(aq) → _Ag₂SO3(s) + _KNO3(aq)
What is the correct complete ionic equation?
K*(aq) + 2503-(aq) + 2Ag+(aq) + 2NO3-(aq) → 2 KNO3(aq) + Ag2SO3(s)
2K+(aq) +SO3-2(aq)+2Ag+(aq) + 2NO3-(aq)→2K+ (aq)+2NO3-(aq) +Ag2SO3(s)
K+aq)+SO3-2(aq)+2Ag+ (aq)+NO3-(aq)→→K+(aq)+NO3-(aq) +Ag2SO3(s)
2K+(aq)+SO3-2(aq)+2Ag+(s)+2NO3-(aq)→2K+(aq) + 2NO3-(aq) + 2Ag(s)+SO2(g)
2K+(aq)+SO3-2(aq)+2Ag+(aq)+2NO3-(aq)→2K+(aq) +2NO3-(aq) + 2Ag+(s)+SO3- ²(s)
2K+(aq) +S(s)+O₂ -2(aq)+2Ag+(aq)+N₂(g) +2O₂-(aq)→2K+(aq)+2NO3-(aq)+A82SO3(s)-

Physical Chemistry
GeneralWhen 5.58 g O₂ react by the following balanced equation, 5.58 g H₂O are formed.
What is the percent yield of the reaction?
2 H₂(g) + O₂(g) → 2 H₂O(l)
Select the correct answer below:
11.1%
38.9%
88.9%
100.0%

Physical Chemistry
GeneralAn analytical chemist is titrating 104.7 ml. of a 0.3500 M solution of trimethylamine ((CH₂), N) with a 0.3400 M solution of HNO3. The pK, of trimethylamine is 4.19. Calculate the pH of the base solution after the chemist has added 127.3 mL of the HNO; solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added.
Round your answer to 2 decimal places.

Physical Chemistry
GeneralFrom the following balanced equation,
4 NH3(g) + 5 O₂(g) → 4 NO(g) + 6H₂O(l)
how many grams of H₂O can be formed from 5.78 g O₂?
Select the correct answer below:
2.71 g
3.15 g
3.90 g
8.56 g

Physical Chemistry
GeneralTo study a marine organism, a biologist prepares a 1.00-kg sample to simulate the ion concentrations in seawater. She mixes
26.5 g of
NaCl,
2.40 g of
MgCl2
3.35 g of
MgSO4,
1.20 g of
CaCl₂,
1.05 g of
KCI,
0.315 g of
NaHCO3, and
0.098 g of
NaBr in distilled water. (a) If the density of the solution is
1.025 g/cm³, what is the molarity of each ion? (b) What is the total molarity of alkali metal ions? (c) What is the total molarity of alkaline earth metal ions? (d) What is the total molarity of anions?

Physical Chemistry
GeneralA sample of a pure compound that weighs 56.6 g contains 22.5 g Sb (antimony) and 34.1 g F (fluorine). What is the percent composition of fluorine?
• Report your answer with three significant figures.
Provide your answer below:

Physical Chemistry
GeneralWrite the equations that show what happens when the following solid ionic compounds dissolve
in water:
1. MgCl,
2. Al(NO3)3
3. Sodium carbonate

Physical Chemistry
GeneralHow many moles of calcium hydroxide are needed to generate 6.76 moles of water, according to the following equation:
3Ca(OH)2 + 2H3PO4 → Ca3 (PO4)2 + 6H₂O
• Report your answer using three significant figures.
Provide your answer below:

Physical Chemistry
GeneralWhat is the empirical formula of a compound that contains 10.0 g carbon, 1.68 g hydrogen, and 13.4 g oxygen?
Provide your answer below:

Physical Chemistry
GeneralA chemist titrates 90.0 mL of a 0.0895 M ethylamine (C₂H5NH₂) solution with 0.8961 M HCl solution at 25 °C. Calculate the pH at equivalence. The pK, of ethylamine is 3.19.
Round your answer to 2 decimal places.
Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HCI solution added.

Physical Chemistry
GeneralA patient comes into the ER complaining of chronic vomiting over the past two
days depleting his stomach of acid. His body adjusts by moving H3O+ from his
blood plasma to his stomach, this raises his blood pH. Is he suffering from
acidosis or alkylosis and w hat will happen to his respiratory rate?
Acidosis; respiration rate decreases
He is neither acidosis or alkylosis; respiratory rate is unaffected
Alkylosis; respiration rate decreases
Alkylosis; respiration rate increases
Acidosis; respiration rate increases

Physical Chemistry
GeneralA chemist mixes 24.0 g H₂ with 8.0 g N2. Assuming the reaction goes to completion, which
reactant should she use to calculate the yield?
3H₂ + N₂ → 2NH3

Physical Chemistry
GeneralOne molecule of bromine (Br₂) and two molecules of potassium chloride (KCI) combine in a reaction. How many atoms a the products?
four
two
three
six

Physical Chemistry
GeneralWhat is the empirical formula of a compound that is 44.4% carbon, 3.8% hydrogen and 51.8% nitrogen by mass?
Select the correct answer below:
CH4N2
C5H5N
CHN
C10HN₂

Physical Chemistry
GeneralFrom the following balanced equation,
4 NH3(g) + 5O₂(g) 4 NO(g) + 6H₂O(l)
how many grams of O₂ had to react to form 3.78 g H₂O?
Select the correct answer below:
2.55 g
4.54 g
5.59 g
8.06 g

Physical Chemistry
GeneralPart B
Calculate the mass of water produced when 2.44 g of butane reacts with excess oxygen.
Express your answer to three significant figures and include the appropriate units.

Physical Chemistry
GeneralThe empirical formula of ethylene, a fruit-ripening gas, is CH₂. The molecular mass of ethylene is 28 amu. What is themolecular formula of ethylene?
Provide your answer below:

Physical Chemistry
GeneralA sample of a pure compound that weighs 64.7 g contains 25.7 g Sb (antimony) and 39.0 g F (fluorine). What is the percent composition of fluorine? Report your answer with three significant figures. ●

Physical Chemistry
GeneralWhich of the follow is the correct net ionic equations for the following potential precipitation reaction: AgNO3(aq) + NaCl(aq) →
Ag + CI----> AgCl
AgNO3(aq) + NaCl(aq)--->AgCl (aq) + NaNO3 (aq)
Ag+ (aq) + Cl- (aq) → AgCl(s)
Na+ (aq) + NO3- (aq) → NaNO3(s)

Physical Chemistry
GeneralWhat mass (in grams) of aspirin (CH.O4) is produced from 57.6 g of CH.Os assuming 95.0% yield from the reaction below?
C7H6O3 (s) + C4H6O3 (1)→ C9HsO4 (s) + HC2H3O2 (aq)

Physical Chemistry
GeneralWhich of the following is NOT part of the effects of hypoxia?
All of the answers are effects of hypoxia
Dilation of peripheral vessels in arms, legs, hands, and feet
Increased heart rate
Increased breathing rate
Constriction of vessels in lungs

Physical Chemistry
GeneralWhich of the following is most likely to form a +2 ion?
A) K
B) Sc
C) AI
D) O
E) Mg

Physical Chemistry
GeneralWhat is the net ionic equation of the reaction of BeCl2 with NaOH?
Express you answer as a chemical equation including phases.

Physical Chemistry
GeneralHow many moles of H₂O form when 3.2 mol of O2 reacts?
Express your answer to two significant figures and include the appropriate units.

Physical Chemistry
GeneralWhen the equation is balanced with its lowest whole-number coefficients, will be the coefficient in front of CuCl₂?
Cu(NO3)2 + AICI3 → CuCl2 + Al(NO3)3
Select one:
a. 4
b. 3
C. 1
d. 2
![An aqueous solution of phosphorous acid is mixed with an aqueous solution of a Group 2 metal hydroxide (metal has a +2 charge). Write and balance the chemical equation. In the balanced chemical equation: • what is the coefficient in front of water? [Select] • which of the following is a spectator ion in the reaction? [Select]](https://media.kunduz.com/media/sug-question/raw/55862257-1659285809.2620227.jpeg?w=256)
Physical Chemistry
GeneralAn aqueous solution of phosphorous acid is mixed with an aqueous solution of a Group 2 metal hydroxide (metal has a +2 charge). Write and balance the chemical equation. In the balanced chemical equation: • what is the coefficient in front of water? [Select] • which of the following is a spectator ion in the reaction? [Select]

Physical Chemistry
GeneralMagnesium oxide can be made by heating magnesium metal in the presence of oxygen. The balanced equation is: 2Mg(s) + O₂ → 2MgO(s), When 150 g of Mg is allowed to react with 250 g O₂, 130 g Mgis collected. Determine the limiting reactant (LR) and percent yield of MgO.
LR:MgO, % yield 67%
LR:Mg, %yield 192%
LR:Mg, % yield 52%
LR: 02, %yield 21%

Physical Chemistry
GeneralAll of the following are true of an oxidation-reduction reaction except:
a. The sum of the charges for the products must be greater than the sum of the charges for the
reactants
b. A metal's redox activity can be predicted by its location on the periodic table
c. It is impossible to reduce a metal without oxidizing a metal
d. The oxidation number of the reducing agent increases