General Questions and Answers

Physical Chemistry
GeneralComprehension XII A volume of 18 ml of a gaseous mixture consisting of a gaseous organic compound A and just sufficient amount of oxygen is required for complete combustion yielding on burning 8 ml of CO 12 ml of water vapour and 4 ml of N All volumes are measured at the same temperature and pressure The compound A contains only carbon hydrogen and nitrogen 34 How many volumes of oxygen are required for 36 complete combustion a 4 ml c 7 ml b 14 ml d 11 ml 35 What is the molecular formula of the compound b C H N d C H N a CH N c C H N What volume of H gas measured at the same temperature and pressure is needed for complete reduction of the same volume of compound A a 4 ml c 28 ml b 8 ml d 14 ml

Physical Chemistry
General44 Extraction of zinc from zinc blende is achieved by a electrolytic reduction b roasting followed by reduction with carbon c roasting followed by reduction with another met d roasting followed by self reduction

Physical Chemistry
GeneralWhich of the following statements is true with respect to 57Fe Mossbauer spectra of i FeSO4 7H O ii K Fe CN iii Na Fe CN 5NO 2H O A singlet in i doublet in ii and iii B singlet in ii doublet in i and iii C singlet in i and iii doublet in ii D doublet in i ii and iii

Physical Chemistry
GeneralWhich of the following is not a correct match A Zn s CuSO4 aq ZnSO4 aq Cu s Displacement reaction B CaO s H O 1 Ca OH 2 aq Heat Combustion reaction C D 2Pb NO3 2 s reaction Heat 2P bO s 4NO2 g O2 g Decomposition Na2SO4 aq BaCl aq BaSO4 s 2NaCl aq Precipitation reaction

Physical Chemistry
GeneralTwo liquids A and B form a non ideal solution which obey the equation po weals When equi molar mixture of A and B distilled find P P 3 P P x 2 P P x the mole fraction of B when this mixture will have a single boiling point TRAA dT dxB I was sealed and heated

Physical Chemistry
GeneralIn a clinical laboratory a sample of urine containing 0 18 g of urea NH CONH2 was treated with an excess of nitrous acid The reaction of urea with nitrous acid is as follows NH CONH2 2HNO2 CO2 2N2 3H O The gases formed were passed through an aqueous sodium hydroxide solution and the final volume after passing through aqueous sodium hydroxide was measured at STP What is the mass of the measured volume of the gas A 0 120 g B 0 168 g

Physical Chemistry
Generalof ferrous ammonium su 3 92 g dissolved in 100m of water 20ml of this folution oquine 18m of potassium permanganate during tration for Complete oxidation The weight KMnoy present in 1lit of the solution is M cl Ferrous ammonium sulphate 392 KMnO 8 034 13 479 3 4769 CC 14 769 9 34 789

Physical Chemistry
GeneralThe first line contains an integer T the number of test cases Then the test cases follow Each test case contains a single line of input three integers A B C Output Format For each test case output in a single line the answer I if it s possible to go out with a pair of shoes and 0 if not Constraints 1 T 8 0 A B C 1 Sample Input 1 3 101 Sample Output 1

Physical Chemistry
General27 Arrange the following chloroarenes in increasing order of their reactivity in nucleophilic substi tution to form their corresponding phenols NO I O N A II V III IV L B II V III I IV C I III IV V II D I IV III V II CI NO NO O N 0 IV O N V

Physical Chemistry
GeneralWhich is NOT related to the element mercury O They all are related to mercury O Can cause mental retardation O Can damage the brain and kidneys Can be absorbed through skin contact O Is denoted as Hg on the periodic table

Physical Chemistry
General2AL 6HCL A 3H g i Identify A ii Idenfity A is soluble salt or insoluble salt Select an answer A B 1 Aluminium chloride II Soluble salt C 1 Aluminium hydride II Insoluble salt D 1 Aluminium chloride II partially soluble salt 1 Aluminium hydride II Soluble salt

Physical Chemistry
GeneralConsider the following reactions R Cu HNO3 dil R Cu HNO3 conc R3 Zn HNO3 dil R4 Zn HNO3 conc Identify the correct statement s In only two reactions same oxide of nitrogen is produced The oxide of nitrogen obtained in R3 is also obtained by the stro heating of NH4NO3 In reaction R and R3 the oxide of nitrogen produced is colourles In reaction R4 the oxide of nitrogen is acidic and colourless

Physical Chemistry
GeneralStibine is a hydride of element E and conjugate base of Stibonium Which of the following is are correct statements for Stibine Bond angle of stibine is less than that of ammonia Stibine is less stable than arsine Element E can be As as well as Sb Stibine is more stable and requires stronger heating to decompose

Physical Chemistry
GeneralFor the reaction A 3B AH ve g g Effect 1 Effect II Effect III P Partial pressure time In the above graph partial pressure versus time is plotted Which of the following is true Forward shift is represented by effect III 2K is only change in effect III 3 Volume of the vessel remains the same in effect 1 and effect II 4 Partial pressure of B at new equilibrium in case of effect III and effect I is higher than that equilibrium

Physical Chemistry
General1 Haemoglobin contains 0 334 of iron by weight The molecular weight of haemoglobin is approximately 67200 The number of iron atoms Atomic weight of Fe is 56 present in one molecule of haemoglobin is 1 4 2 6 3 3 4 2

Physical Chemistry
General1 With reference to Rutherford s Scattering Experiment answer the following questions A What was the thickness of gold foil used i n the experiment B With which particles was the gold foil bo mbarded
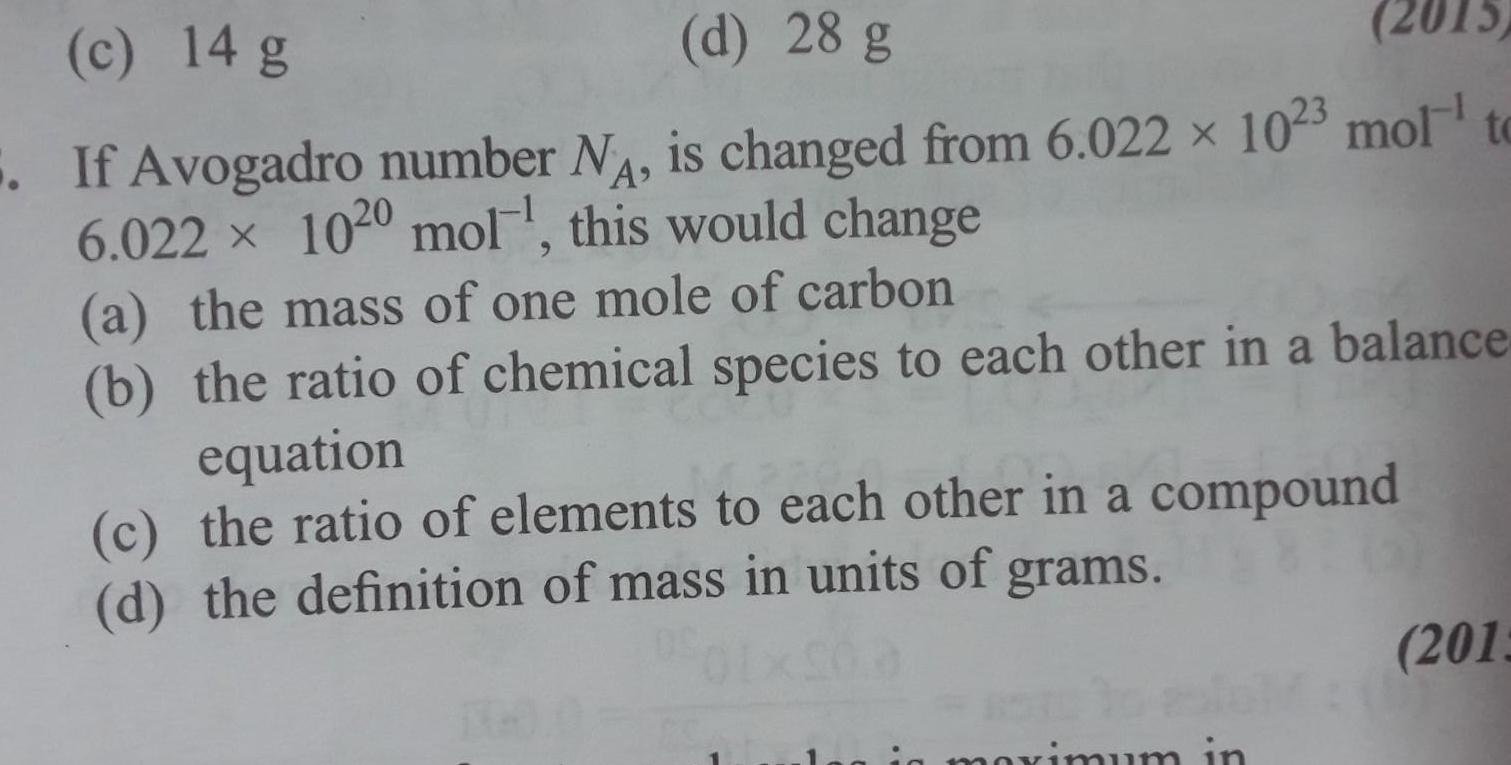
Physical Chemistry
Generalc 14 g d 28 g 2015 If Avogadro number N is changed from 6 022 x 1023 mol to 6 022 1020 mol this would change a the mass of one mole of carbon b the ratio of chemical species to each other in a balance equation c the ratio of elements to each other in a compound d the definition of mass in units of grams maximum in 2013

Physical Chemistry
General2 AH ve AS ve There are many substance for example CO NO etc do not follow third law of thermo dynamics because 1 these substances are polar 2 these substance do not form crystal at absolute zero temperature 3 these substances do not fulfill the perfectly ordered arrangement at absolute zero 4 these substance follow the second law of thermodynamics 3 82 C A 1 2 3 4

Physical Chemistry
GeneralHow much product will be formed if each of the reactants used is 100 g in quantity Atomic weight Al 27g mol and Atomic weight of Cl 35 5 g mol Max score 4 Neg score 1 150 g 125 g

Physical Chemistry
GeneralVolume strength of H O is 5 6 V then whic statement is are true 1 1 mL of H O given sample liberate 5 6 mL O at N T P 2 Normality of solution is 1 N 3 Molarity of solution is 2 M 4 7 g of H O2 are present per 1000 mL solution

Physical Chemistry
GeneralFind out D in the following sequence of reactions Br Fe Zn HCI D C H B C A CH CH CCI CCI3 Br Br Br

Physical Chemistry
Generalsolution of silver nitrate Max score 2 Neg score 0 5 copper will remain unreacted due to the presence of nitrate ions the solution will remain colourless

Physical Chemistry
General3 H is limiting reagent 9 40 g water is formed Equal volume of N and H react to form ammonia under suitable condition then the limiting reagent is N 3H 2NH3 2 N 4 No one reactant is limiting reagent 1 H 3 NH How many grams of calcium oxide is obtained on heating 100 g of CaCO3 s

Physical Chemistry
General1803080201030001 202 Question No 18 40 The most probable distribution of N indistringuishable particles various energy levels according to the Fermi Dirac statistics 696 20 AO gi 1 BG 1 co Si BON x G 1 6962020064696 2020064 02 969792 20064 90 Si x BT 1 8 DO n x BG 1 2020064696 202006 9691 4696 20

Physical Chemistry
Generaliv Without constructing the actual matrices construct the set of characters of a representation of a point group of the following molecules in the basis set given against each Molecule Ammonia Basis Set The set of unit vectors stretched along the N H bonds
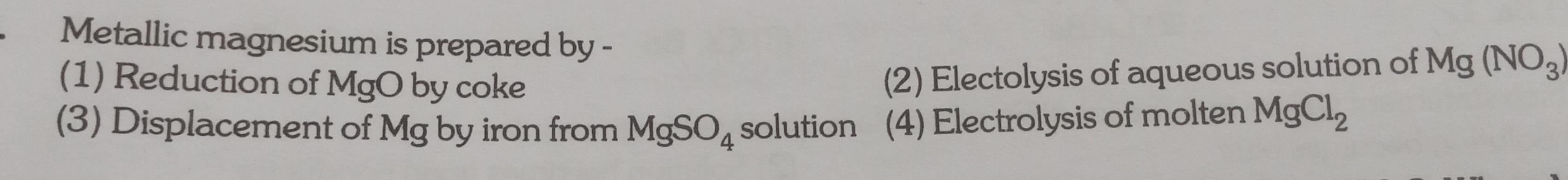
Physical Chemistry
GeneralMetallic magnesium is prepared by 1 Reduction of MgO by coke 3 Displacement of Mg by iron from MgSO4 solution 4 Electrolysis of molten MgCl 2 Electolysis of aqueous solution of Mg NO3

Physical Chemistry
Generalwhen iron spoon is electroplated with copper which of these represent reduction reaction skipped B C D Cu aq 2e Cu s Cu s Cu aq 2e Fe aq 2e Fe s Fels Fe aq 2e

Physical Chemistry
General4 The values of Ksp of CaCO3 and CaC204 are 4 7 10 and 1 3 x 10 9 respectively at 25 C If the mixture of these two is washed with water what is the concentration of Ca2 ions in water 1 7 746 10 5 M 3 6 856 10 5 M 2 5 831 x 10 5 M 4 3 606 10 5 M

Physical Chemistry
Generaldt dt dt a a EXAMPLE 5 When 50 ml of 2M solution of N O5 was heated 0 28 L of O at NTP was formed after 30 mi Calculate the concentration of N O5 at that time and also find the average rate of reaction

Physical Chemistry
General28 At temperature above 1073 K coke can be used to reduce Fe0 to Fe How can you justify this reduction with Ellingham diagram s Using Ellingham diagram we observe that at temperature greater than 1073 K We know that according to Ellingham diagram compound having lower A GS undergo its formation AGIC CO AG Fe FeO Hence coke can reduce FeO to Fe

Physical Chemistry
General2012 2 A closed vessel with rigid walls contains 1 mol of 238U and 92 1 mol of air at 298 K Considering complete decay of 238U 92 to 206Pb the ratio of the final pressure to the initial pressure 82 of the system at 298 K is

Physical Chemistry
GeneralA solid compound X on heating gives CO gas and a residue The residue mixed with water forms On passing an excess of CO through Y in water a clear solution Z is obtained On boiling Z compour X is reformed The compond X is 1 CaCO3 2 Na CO 3 K CO 4 Ca HCO3 2

Physical Chemistry
GeneralBe sure to answer all parts Give the oxidation number of sulfur in the following a SOCI select b H S select c H SO3 select d Na S select TINTOFF 4 3 2

Physical Chemistry
General37 The density of argon face centered cubic cell is 1 83 g cm at 20 C What is the length of an edge a unit cell Atomic mass Ar 40 b 0 569 nm a 0 599 nm c 0 525 nm 38 The density of nickel face centered cubic cell is 8 94 g cm3 at 20 C wh d 0 551 nm 1 1

Physical Chemistry
General4 4 0 m 1 solution of H O2 completely reacts with 10ml of 0 5 molar KMnO4 solution o specific gravity 0 4316 as per reaction 2KMnO4 3H SO4 5H2O2 K2SO4 2MnSO4 502 8H O If volume strength of H O2 is x then find the value of 3x 7

Physical Chemistry
GeneralIdentify the incorrect match Name A Unnilunium B Unniltrium C Unnilhexium D Unununnium a A i c C iii IUPAC Official Name i Mendelevium ii Lawrencium iii Seaborgium iv b B ii d D iv NEET 2020 Darmstadtium

Physical Chemistry
GeneralFind the total number of different possible combination product Considering only combination of radical but not disproportionation of radical for following Wurtz reaction Na H3C CI H3C CH CI H3C CH CH CI Dry ether Backspace

Physical Chemistry
GeneralQ 55 The density of solid Argon is 1 6 ml at 233 C If the Argon atom is assumed to be sphere of radius 1 5 x 108 cm then the of solid Argon is apparently occupied Take N 6 x 1023 Atomic mass of Ar 40

Physical Chemistry
GeneralA 2 650x10 2 M solution of glycerol C3H O3 in water is at 20 0 C The sample was created by dissolving a sample of C3Hs Os in water and then bringing the volume up to 1 000 L It was determined that the volume of water needed to do this was 998 9 mL The density of water at 20 0 C is 0 9982 g mL
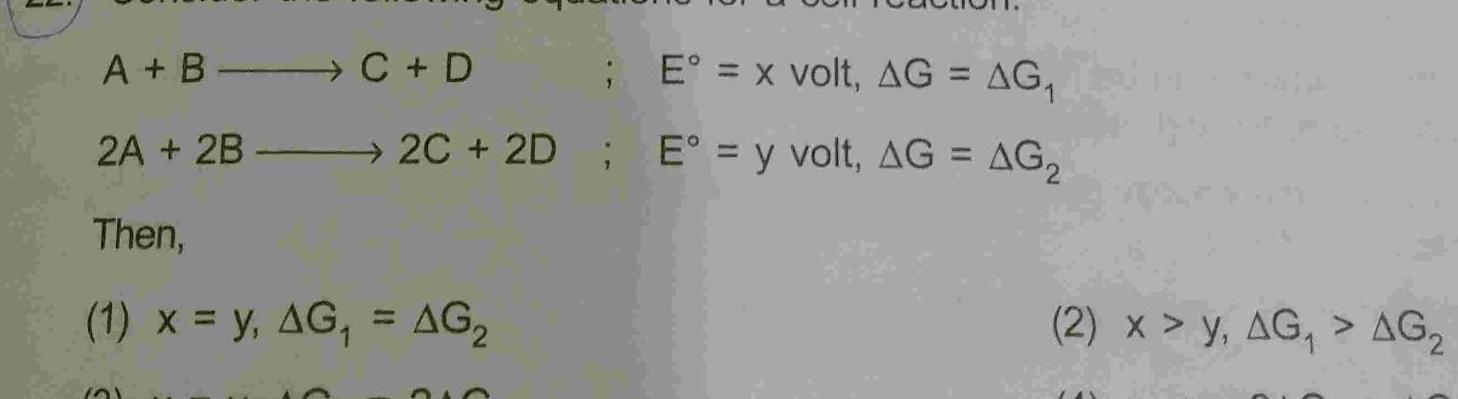
Physical Chemistry
GeneralA B C D 2A 2B Then E x volt AG AG 2C 2D E y volt AG AG 1 x y AG AG 21 210 2 x y AG AG
Physical Chemistry
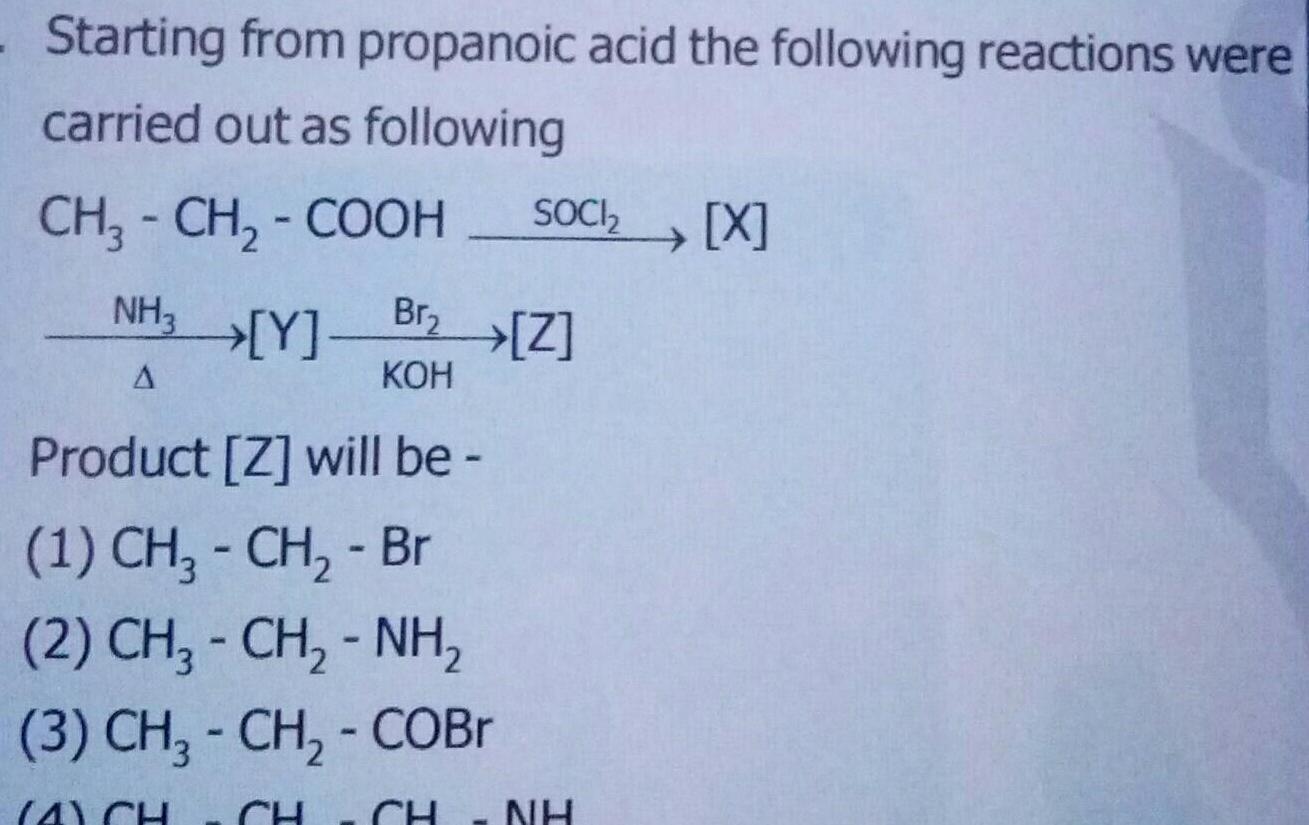
GeneralStarting from propanoic acid the following reactions were carried out as following CH3 CH COOH NH3 Y A SOCI X Br Z KOH Product Z will be 1 CH CH Br 2 CH CH NH 3 CH CH COBr 4 CH CH NH

Physical Chemistry
General6 An electron is moving in 3rd orbit of Hydrogen atom The frequency of moving electron is 2 7 3 1014 rps 4 7 3 1010 rps 1 2 19 1014 rps 3 2 44 1014 rps hanical Modell

Physical Chemistry
Generalalculate the amount in gram of the sulphate ions olution MM 7200 IIT JEE Find the weight of sodium bromate and mola solution necessary to prepare 85 5 mL of 0 solution when the half cell reaction is ab BrO3 6H 6e Br 3H O4 Find the weight as well as molarity if the h reaction is 2BrO3 12H 10e Br 6H O tulo I uncin IIT JE A sample of hydrazine sulphate N H SO4 was d in 100 mL of water and 10 mL of this solution wa with excess of ferric chloride solution and wa complete the reaction Ferrous ion formed was e and it required 20 mL of M 50 potassium perm solution Estimate the amount of hydrazine sulp tog de Admi I of the solution

Physical Chemistry
GeneralThe ammonia prepared by treating ammonium sulphate with calcium hydroxide is completely used by NiCl2 6H O to form a stable coordination compound Assume that both the reactions are 100 complete If 1584 g of ammonium sulphate and 952g of NiCl 6H O are used in the preparation the combined weight in grams of gypsum and the nickel ammonia coordination compound thus produced is Atomic weights in g mol H 1 N 14 O 16 S 32 Cl 35 5 Ca 40 Ni 59 29

Physical Chemistry
GeneralAccording to the real gas equation Z 1 for an ideal gas and Z is variable for a real gas Suppose in order to easy our calculations we fixed Z 1 for a real gas and for ideal gas Z will become variable Z vs P for an ideal gas will be similar to ZI P P b d K p L P P

Physical Chemistry
GeneralWhich of the following statements is are correct C Reversible adiabatic process is isoentropic process for irreversible adiabatic compression is greater than zero AS system S for free expension in zero for irreversible is greater than zero AS system surrounding compression isothermal

Physical Chemistry
General6 11 2 L H and 11 2 L Cl2 allowed to react at STP and gas produced dissolved in 1 L water The volume of given solution which can be neutralized completely by 500 ml of 0 2 M KOH is 1 100 mL 2 200 mL 4 250 mL 3 500 mL

Physical Chemistry
GeneralSolvelancer Test The formation of AB takes place according to the given reaction A2 B2 2AB 20 kJ The enthalpy of formation of AB is Solvelancer Test a 10 kJ mol b 20 kJ mol c 10 kJ mol d 20 kJ mol

Physical Chemistry
General9 25 Options 11 22 3 3 44 0 29 The molar conductance of acetic acid is 40 S em mol If the dissociation constant is 1 x10 mol L then the concentration of acetic acid is ANAL 90 S cm mol ANC 120 S cm mol and AC 430 Sem mol Options 11 1 2 9x 10 mol L 3 400 Sem mol 4 40 Sem mol 9 x 10 mol L 4 The technique in which the liquid boils when the sum of vapor pressure due to organic liquid P and that due to water P becomes equal to the atmospheric pressure P is 1 Sublimation 2 Fractional distillation 3 Steam distillation 4 Evaporation Quention Type MCQ Que101900091278 Optan 11D 100soes Option 210 1504 Opton 310 19095047 Option 4 10 11 Stat Answered Chosen Option 3 Question Type MCQ Question 10 1908891249 Option ID 1908804029 Option 2 10 1908894930 Option 3 ID 1908894931 Option 4 ID 1900804932 Status Answered Chosen Option 3

Physical Chemistry
GeneralGiven that Efe2 Fe Fe Fe 0 44V Eg Fe Fe If Fe Fe and Fe solid are kept together then 1 Fe increases 2 Fe decreases 3 Fe Fe remain unchanged 4 Fe decreases 0 77V