General Questions and Answers

Physical Chemistry
GeneralMatch the transformations in column I with appropriate options in column II Column I Column II A CO s CO g B CaCO s CaO s CO 9 3 C 2H H g D P white solid P red solid p phase transition q allotropic change r AH is positive s AS is positive t AS is negative 2011 ntholny of formation at 298 K is

Physical Chemistry
GeneralWrite IUPAC name of the given compound Solution The locant in this molecule is given as 2 9 CH CH CH CH CH C CH CH CH CH CH CH CH CH CH CH CH 3 CH 21 CH CH CH3 TT 8 7 6 CH CH C CH CH 51 CH CH CH CH CH CH CH CH CH The IUPAC name of the compound is given as CH 4 3 2

Physical Chemistry
General154 180 1 DIBAL H ii H O CH CH CH CH CH CN Major product formed in the given reaction is CH CH2 CH2 CH CH2 CH NH CH CH2 CH2 CH CH CHO CH CH CH CH2 CH CHO CH CH CH CH CH CH NH

Physical Chemistry
General12 mole C s reacts with 9 mole of O g to form CO g and CO g If no one reactant remains then calculate the density of final gas mixture in g L at temperature and 1 642 atm pressure R 0 0821 lit atm mole K

Physical Chemistry
GeneralIdentify the properties of the gases produced in the following reactions Gas 1 evolves when iron reacts with sulphuric acid Gas 2 evolves when ferric carbonate reacts with sulphuric acid A Gasl Burns in air Crackling sound Gas 2 It doesn t burn in air B Gasl Burns in air No sound Gas2 Turns lime water milky C Gasl Turns lime water milky Gas 2 Burns in air Pop sound D Gasl It doesn t burn in air Gas 2 Crackling sound Q12 OPTIONS

Physical Chemistry
GeneralA 10 mg effervescent tablet containing sodium bicarbonate and oxalic acid releases 0 25 ml of CO at T 298 15 K and p 1 bar If molar volume of CO2 is 25 0 L under such condition what is the percentage of sodium bicarbonate in each tablet Molar mass of Nah undat co 84 g mol 1 33 6 Services Limited JEE Main 2019 2 8 4 4 16 8

Physical Chemistry
GeneralSolveLancer Test Millerite is an ore of Metal M Give the atomic number group number and valence electrons respectively for Metal M SolveLancer Test a 26 8 8 b 28 10 12 c 26 8 10 d 28 10 10

Physical Chemistry
GeneralBasic reaction is that when metal reacts wit h acid it forms hydrogen and example was given that when metal copper reacts with ac id it forms hydrogen but this example is con tradicted in the explanation which given in t he solution of a question in the test questio n was that which reaction produces flamma ble gas One option was the reaction of Cop per and acid and other option was potassiu m and acid Option of reaction of Copper an d acid was told to be wrong polished off so i t has contradicted the video statement Plea

Physical Chemistry
GeneralQuestion 29 Which of the following is a valid set of quantum numbers On 4 1 3 m 3 On 3 1 2 m 3 On 3 1 2 m 3 On 4 1 4 m 3

Physical Chemistry
Generalplease explain the solution of this question i have read the solution given for this question it was give In that if we take more salts more fluid is retained in our b ody how is this related to high BP another answer said that osmotic pressure increases causi ng rupture of RBCs i dont understand how increase in os motic pressure would cause rupture of RBCs please explain clearly ouluun 1 mypotom o 41 Doctors advises patients of high blood pressure to take less quantity of common salt why Op

Physical Chemistry
General7 25 ml of the given HCI solution requires 30 mL of 0 1 sodium carbonate solution What is the of Aaka volume of this HCI solution required to titrate 30 mL of 0 2 M aqueous NaOH solution 1 25 mL 3 50 mL JEE Main 2019 2 12 5 mL 4 75 mL

Physical Chemistry
Generalas well D Write true or false 1 Accidents can occur anytime 2 We must follow safety rules to prevent accidents 3 We should keep our toys scattered in the room 4 If a person has some injury first aid should be given immediately 5 We should take medicines without consulting the doctor E Tick the rules which are safe

Physical Chemistry
GeneralHydrolysis product of which of the following is used for blocking the polymeric chain of silicones MeSiCl 3 Me SiCl2

Physical Chemistry
GeneralConsider the following redox reaction H O AX BY HA YO BX2 unbalanced It is also known that oxidation number of X is 2 and neither X nor water is involved in the redox process In compound BY B is cation and consider its oxidation number 4 Select the incorrect option O The element A is undergoing reduction The element B is undergoing reduction The element B is undergoing oxidation The element Y is undergoing oxidation

Physical Chemistry
GeneralA colourless salt when treated with con H SO4 evolve a colourless gas with strong odour which gives white dense fumes with glass rod dipped ammonia The anion of the salt can be A CO B CI C SO D NO 3

Physical Chemistry
GeneralTrial 3 Volume of liquid ml 10 9 8 REPORT Trial 4 Mass of graduated cylinder and liquid g SUMMARY 0 01 Saved

Physical Chemistry
GeneralThere were 35 students in a hostel If the number of students is increased by 7 the expenditure on food increase by Rs 42 per day while the average expenditure of students is reduced by Rs 1 What was the initial expenditure on food per day a Rs 400 c Rs 442 d Rs 420 b Rs 432 e None of these

Physical Chemistry
GeneralIf we consider that 1 6 in place of 1 12 mass of carbon atom is taken to be the relative atomic mass unit the mass of one mole of a substance will AIEEE 2005 a be a function of the molecular mass of the substance b remain unchanged c increase two fold d decrease twice

Physical Chemistry
General174 For an equilibrium H O s of the following statements is true A The pressure changes do not affect the equilibrium H O 0 which B More of ice melts if pressure on the system is increased C More of liquid freezes if pressure on the system is increased D The pressure changes may increase or decrease the degree of advancement of the reaction depending upon the temperature of the

Physical Chemistry
GeneralSolve lancer test Choose the correct statement Solve lancer Test a Addition of Hydrogen cyanide to carbonyl compound b preferably occurs in both acidic as well as basic medium Carbonyl compounds say ketone on treatment with Grignard reagent produces 2 alcohols c Carbonyl compounds form crystalline addition compounds on treatment with NaHSO3 d None of the above statement is true

Physical Chemistry
GeneralA sample containing 1 mol KHC O4 H C O4 is titrated with different reagents Select correct statement s 1 mol of KOH is used 3 2 moles of Ba OH 2 is used 4 5 mol of KMnO4 is used in faintly alkaline medium

Physical Chemistry
GeneralThe slope of straight line ploted between log a x and t is 51 log a x equal to 0 03 The rate constant of reaction is 1 6 9 x 10 2 2 6 9 3 0 69 4 6 9 x 10 fuffranian 1 6 9 x 10 2 6 9 3 0 69 4 6 9 x 104

Physical Chemistry
GeneralName the compound shown below 5 ethyl 4 methyl 2 heptyne O 4 ethyl 5 methyl 2 heptyne 4 methyl 3 ethyl 5 heptyne 5 methyl 4 ethyl 2 heptyne 4 methyl 5 ethyl 2 heptyne 3 ethyl 4 methyl 5 heptyne

Physical Chemistry
GeneralSolveLancer Test Write the complete combustion reaction of butane What is the sum of whole number stochiometric coefficients involved in the balanced chemical equation SolveLancer Test a 30 b 31 c 32 d 33

Physical Chemistry
GeneralWhich of the following is a buffer solution 1 500 ml of 0 1 N CH COOH 500 mL of 0 1 N NaOH 2 500 ml of 0 1 N CH COOH 500 mL of 0 1 N HCI 3 500 ml of 0 1 N CH COOH 500 mL of 0 2 N NaOH 4 500 ml of 0 2 N CH3COOH 500 ml of 0 1 N NaOH Answe

Physical Chemistry
GeneralThe position vector of a particle is r a cos oot i a sin oot j The velocity vector of the particle is a Parallel to position vector b Perpendicular to position vector c Directed towards the origin d Directed away from the origin


Physical Chemistry
GeneralThe electronic configuration of Cu II is 3d whereas that of Cu I is 3d 0 Which of the following is correct Cu ll is more stable CORRECT ANSWER Cu l and Cu II are equally stable N Cu ll is less stable INCORRECT Stability of Cu I and Cu II depends on nature of copper salts

Physical Chemistry
GeneralDetermine the total heat in Joules U required for 15 g of ice to change from a temperature of 20 C to 125 C Heat of Vaporization Melting point C 0 Boiling Point C 100 Temperature C Heat of Fusion 6 02 40 7 C solid 37 6 C liquid c 75 4 C vapor re 33 1

Physical Chemistry
GeneralChoose the incorrect statement a Vitamin B is also known as thiamine b Riboflavin is another name for vitamin B c Vitamin E is also called pyridoxine d Ascorbic acid is another name for Vitamin C

Physical Chemistry
GeneralHow many mL of 0 250 M KMnO4 are needed to react with 3 55 g of Iron II sulfate FeSO4 The reaction is as follows 10FeSO4 aq 2KMnO4 aq 8H SO4 aq 2MnSO4 aq K SO4 aq 5Fe SO4 3 aq 2H O 1

Physical Chemistry
General4 1 8 mole lit 3 0 9 mole lit 4 1 8 mole lit 3 A certain zero order reaction has k 0 025 M s for the 48 f 0 025 M S disappearance of A What will be the concentration of A after 15 seconds if the initial concentration is 0 50 M 1 0 5 M 2 0 375 M A 1 0 5 M 2 0 3 3 0 125 M 4 0 06 M 3 0 125 M 4 0 In the reaction NH NO aq N 0 HO

Physical Chemistry
GeneralSolveLancer Test Consider the reaction given below BaCl aq 2AgNO3 aq 2AgCl Ba NO3 2 aq 1 56 g of BaCl2 limiting reagent reacts to give 2 01 g of AgCl calculate the percentage yield of the reaction Given Molar mass of Ba and Ag is 137 g mol and 108 g mol respectively SolveLancer Test a 90 b 93 4 c 84 5 d 88

Physical Chemistry
GeneralSolveLancer Test During crystallization the filtrate that contains the impurities and very minute quantity of the compound is called as SolveLancer Test a Mother liquor b Impure filtrate c Crystallized liquid d Salt induced liquid

Physical Chemistry
GeneralThe manganate and permanganate ions are tetrahedral due to 1 The T bonding involves overlap of p orbitals of oxygen with d orbitals of manganese 2 There is no bonding 3 The T bonding involves overlap of p orbitals of oxygen with p orbitals of manganese 4 The T bonding involves overlap of d orbitals of oxygen with d orbitals of manganese

Physical Chemistry
GeneralWhich of the following sets of quantum numbers is not allowed SOOJU I show Donje DJ woodwa d Options 1 n 4 1 3 m 3 s 2 x n 2 1 1 m 0 s 4 3 n 1 1 0 m 0 s AP EAMCET 2020 AP EAMCET 2020

Physical Chemistry
GeneralWe know that metal react with water to give metal oxide and hydrogen but in o ur textbook an equation is given like thi S 2k 2H O 2koH H Similarly sodium react with water to giv e sodium hydroxide Why is it not sodiu m oxide

Physical Chemistry
GeneralConsider the following statements about boric acid I It is a weak monobasic acid II It is not a protonic acid III It acts as a lewis acid The correct statement s among the following is are I only I and II only I and III only I II and III

Physical Chemistry
General40 3733 E Sn HCI 1 O Conc HNO3 Conc H SO4 C 2 Br NaNO HCI 273 278K OH D Br 3 A Br Fe Boiling dil H SO4 NO E O Br B 4

Physical Chemistry
General3 A 1 2 4 A 2 4 The rate constant of the reaction A 2B is 1 0 x 10 mol 47 fferal A afe A atr lit min If the initial concentration of A is 1 0 mole lit what would be the concentration of B after 100 minutes 1 0 1 mole lit B 2 0 2 mole lit 3 0 9 mole lit 4 1 8 mole li 1 0 1 mole 2 0 2 moles 3 0 9 mole

Physical Chemistry
GeneralIn acidic medium H O2 converts PbS into X The behaviour of H O2 in the reaction and the product X so formed respectively are Reducing PbSO4 Oxidising PbSO4 Reducing SO2
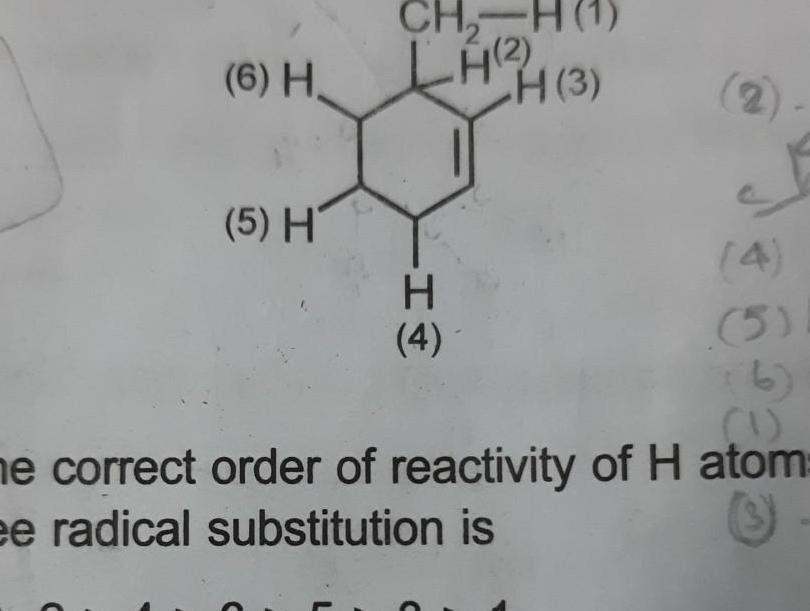
Physical Chemistry
General6 H 5 H CH LH 4 3 H 4 4 5 1 ne correct order of reactivity of H atom ee radical substitution is

Physical Chemistry
GeneralHow would you balance the following oxidation half reaction Fe s Fe aq You can either write out the balanced half reaction or simply state what and how many of somet you would add to either the product or reactant side

Physical Chemistry
General78 3 g of a volatile metal chloride when vapourised occupies volume of 6 72 litre at STP When 10 44 g of the metal chloride was heated with an excess of Mg powder 7 6 g of MgCl was produced along with metal Consider the metal chloride is a monomer even in vapour phase and contain only 1 metal atom per formula Then identify the correct statement s A The atomic mass of metal is 119 u B The oxidation number of the metal is 3 C 4 76 g of metal is obtained on the reaction of above sample of metal chloride with Mg powder D The mass of Mg powder used in the above reaction of metal chloride with Mg is 2 4 g if the Mg powder used is 80 pure

Physical Chemistry
GeneralThe ratio of mass percent of C and H in an organic compound CxHyO is 6 1 If one molecule of the above compound CxHyOz contains half as much oxygen as required to burn one molecule of compound CxHy completely to CO2 and H O The empirical formula of compound CxHyOz is

Physical Chemistry
General16 Calculate enthalpy of formation of C diamond if enthalpy of atomisation of C graphite 710 kJ mot and energy required to break C C bond in diamond solid 354 05 kJ mot A 2 9 kJ mol B 1 9 kJ mol C 3 3 kJ mol D 0 9 kJ mol

Physical Chemistry
GeneralIncorrect statement about graphite is It has layered structure Each layer is composed of planar pentagonal rings of carbon atoms Each carbon atom is sp hybridised It conducts electricity

Physical Chemistry
General16 Ferrous sulphate crystals decompose on heating to give Fe2O3 SO2 and gas X Gas X is Only one correct answer A 02 B SO3

Physical Chemistry
GeneralIn the phenomenon of osmosis through the semipermeable membrane 1 Solvent molecules pass from solution to solvent 2 Solvent molecules pass from solvent to solution 3 Solute molecules pass from solution to solvent 4 Solute molecules pass from solvent to solution

Physical Chemistry
GeneralWhen a man walks on rough horizontal surface then nature and direction of force of friction between shoes and ground A Dynamic forward B Dynamic backward C Static forward D Static backward