General Questions and Answers

Physical Chemistry
General37 At 727 C N g 3H g 2NH g AH 123 77 N NH 3 5R 4R Substance H 3 5R C The heat of formation of ammonia at in kJ mol at 270 a 44 42 b 44 42 c 88 85 d 88 85 a a C 45 C

Physical Chemistry
Generalgives the energy of the electron in orbital d Both a b 39 Consider the following sets of quantum number 1 0 ii iii iv v n 3 2 4 1 3 2 3 0 2 m 0 1 2 1 3 S 1 2 1 2 1 2 1 2 1 2 Which of the following sets of quantum number is not possible a i ii iii and iv b ii iv and v c i and iii d ii iii and iv

Physical Chemistry
General60 Al O is reduced by electrolysis at low potential and high current If 4 0 x 104 amperes of current is passed through molten Al O for 6 hours what mass of aluminium is produced Assume 100 current efficiency At mass of Al 27g mol A 1 3 x 104 g C 8 1 x 104 g B 9 0 x 10 g D 2 4 x 105 g

Physical Chemistry
Generala mixture that possesses chiral center s and is optically active a mixture that possesses chiral center s but is optically inactive None of the above a pure compound that possesses chiral center s but is optically inactive

Physical Chemistry
Generalecre CI O S O OPCI O P O 4 g oxygen expand at STP to its original volume Then work don 1 260 cal 2 180 cal 3 130 cal 4 272 8 cal 7 Which of the following is displaced by Fe 2 Zn 4 All of these Which of the following will not give ether as product 1 Ag 3 Na 68 CH CH I CU ZONS CH 00 ONa JONa CH CH CH So

Physical Chemistry
General47 What amount of energy in KJ is released in the combus 47 tion of 11 6 gm of CH10 9 2CH 9 130 9 BCO 2 0 10H 0AH 5756 KJ 1 575 6 2 287 8 3 182 11 6 TCH109 a difer f K 2CH g 130 g BCO2 g 10H 0AH 5756 KJ 1 575 6 2 287 8 3 182 4 57 56 4 57 56 18 The potential energy of an electron in He is 6 8 eV The 48 Hegfruf 6 8 eva fam

Physical Chemistry
General22 Which of the following statements is wron for gases a Confined gas exerts uniform pressure a the walls of its container in all directions b Volume of the gas is equal to volume c container confining the gas c Gases do not have a definite shape and 3 volume d Mass of a gas cannot be determined by weighing a container in which it i enclosed 1000 29

Physical Chemistry
General114 Which among the following pairs of reactions has the most favourable equilibrium constant P x CH3CH OH NH3 CH3CH O NH4 y CH3OH NH3 CH3O NH Q x CH3CH OH NH3 CH3CH O NH4 y CH3CH OH CH3NH2CH3CH O 1 P x Q y 2 P x Q x 3 P y Q y 4 P y Q x CH NH

Physical Chemistry
General30 A body of mass 1 kg is executing simple harmonic motion Its displacement y cm at t seconds is given by TC Its maximum kinetic energy is y 6sin 100t 4 a 6 J c 24 J b 18 J d 36 J

Physical Chemistry
General42 A particle of mass 1 kg is undergoing S H M for which graph between force and displacement from mean position is shown Its time period in seconds is a 3 C 6 1 5 13 5 F N 13 5 1 5 b 2 3 d 3 x m

Physical Chemistry
GeneralThe activation energy for the forward reaction R P 30 kJ is 40 kJ The activation energy for the reverse reaction is X 70 kJ 10 kJ 70 kJ 10 kJ Solution Answer 2 Ea f 40 Ea b AH 30 AH Ea f Ea b

Physical Chemistry
Generalthen column in a U tube h mb of U tube spring of force constant ation will be ang is assumed to be nstant K when time is noted ot 0 y a cos when time is noted form the extreme position of SHM 1 A body oscillates with SHM according to the equation x TC 5 cos 2 t Its instantaneous displacement at t 4 1 sec is S S com the mean position of SHM 2 b d 5 05

Physical Chemistry
GeneralThe pH value of lemon juice is 3 3 whereas pH of sodium hydroxide is 11 5 So Lemon j uice is acid Sodium hydroxide is a alkaline But We can drink lemon juice which is acidic and can t drink sodium hydroxide which is al kali Why Acid is edible while alkali is non e dible

Physical Chemistry
GeneralOne mole of ideal gas is adiabatically and reversibly 1 compressed to th of its original volume Change 4 in temperature of the gas if initial temperature 27 C given y 1 66 1 100 K 3 449 K 2 200 K 4 350 K the follow

Physical Chemistry
General4 C graphite 4H g CH g 63 Calorific Fuel value of ethane in kJ g if for the reaction 63 2C H 70 4CO 6H 0 AH 745 6 kcal 1 12 4 2 52 3 24 8 4 104 pontaneous 2C 1 3 FAI

Physical Chemistry
General19 According to kinetic theory of gases there are 1 intermolecular attractions 2 molecules which have considerable volume 3 no intermolecular forces of attraction 4 the velocity of molecules decreases for each collision

Physical Chemistry
Generald 3 1 3 A 10 kg metal block is attached to a spring of sp of spring constant 1000 Nm A block is displa from equilibrium by 10 cm and released the maxi acceleration a 10 m s of block is b 100 m s 200

Physical Chemistry
Generalenergy 26 When a spring is stretched by 10 cm the potential e stored is E When the spring is stretched by 10 cm more the potential energy stored in the spring becomes b 4E a 2E c 6E d 10E

Physical Chemistry
GeneralA 0 5 gm sample of solid Fe O3 OF 55 2 purity is dissolved in acid and reduced by heating the solution with zinc dust The resultant solution is cooled and made up to 100 ml 75 ml of this solution requires 17 ml of 0 0167M solution of an oxidant for titration The number of electrons in nearest integer taken up by one molecule of oxidant in the above titration is

Physical Chemistry
GeneralA chiral center with R configuration Orotates the propagation plane of light in counter clockwise direction None of the answers provided Is always an L configuration Is always a D configuration the propagation plane of light in clockwise direction

Physical Chemistry
GeneralWhich characteristic s of rems compound s below is are accurate The chiral centers in miso compounds possess the exact same set of substituents Meso compounds possess chiral centers but the rotations completely cancel each othe out All of the listed characteristics are accurate Meso compounds display zero net rotation of miso compounds has an internal plane of symmetry

Physical Chemistry
General5 A simple pendulum of frequency n falls freely unc gravity from certain height from the ground level frequency of oscillation a Remain unchanged b n 2 c Becomes zero d Becomes infinit

Physical Chemistry
General29 The displacement of a particle executing SHM is given where y is in meter at time t in c 0 25 T by y 10sin 6t 3 d seconds The initial displacement and velocity of the particle are respectively 5 3 m and 30m sec a b 15m and 5 3m sec c 15 3 m and 30m sec C sec 10 3 2 m and 30m sec

Physical Chemistry
GeneralCaustic soda NaOH can be prepared commercially by the reaction of Na CO3 with slaked lime Ca OH 2 How many gram of NaOH can be obtained by treating 1 06 kg of Na CO3 with 1 48 kg of slaked lime Na CO3 Ca OH 2NaOH CaCO3

Physical Chemistry
GeneralThe term Racemic refers to Any mixture of enantiomers that displays no net optical activity A 50 50 mixture of enantiomers that displays no net optical activity Any mixture of diastereomers that displays no net optical activity A 50 50 mixture of diastereomers that displays no net optical activity

Physical Chemistry
GeneralIf enantiomer A has 3 chiral centers a R configuration b R configuration and c S configuration which have specific rotations of 33 0 20 5 and 32 0 respectively what will the observed rotation for enantiomer A be 44 5 degrees 85 5 degrees O 85 5 degrees 445 degrees

Physical Chemistry
GeneralThe correct statements about production of Br are A The pH of the sea water is raised to about 8 5 B The pH of the sea water is lowered to about 3 5 C An excess of chlorine is bubbled through the sea water D Br and BrO3 ions can be converted to Br by oxidation with Cl H SO4 O A and D O A C and D OB C and D A and C

Physical Chemistry
GeneralThe molar mass of NH3 is 17 03 g mol and the molar mass of H O is 18 02 g mol Consider the following already balanced chemical equation 4 4 NH3 g 7 O g 4 NO g 6 H O g What is the conversion factor you would use to convert from grams of NH3 to moles of NH3

Physical Chemistry
General4 What is the phase difference between two simple TC harmonic motions represented by X A sin ot and X A cos oot 6 a C T 6 T b d F m 5 T 3

Physical Chemistry
General28 The displacement of a body executing SHM is given by x A sin 2 t 3 The first time from t 0 when the velocity is maximum is b 0 16 sec a 0 33 sec c 0 25 sec d 0 5 sec The displacement of a particle executing SHM is gir

Physical Chemistry
Generald 1 5 cm 10 A particle is executing SHM Then the graph of velocity as a function of displacement is b Circle d Hyperbola a Straight line c Ellipse

Physical Chemistry
GeneralThe observed rotation of a sample subjected to polarimetry is 0 0 Which statement is accurate about the identity of this sample The sample is a mess compound All of the statements are accurate The sample is a racemic mixture The sample is achiral

Physical Chemistry
GeneralFrom the following energy levels of hydrogen atom the values of E and E3 in J are respectively I E E3 E 0 545 10 8 J E 2 18 10 18 J TS EAMCET Engg 2019 0 242x10 A 1 0 242 10 19 O A 1 18

Physical Chemistry
GeneralIs also of R configuration Is also of S configuration Rotates the propagation plane of polarized light in clockwise fashion Is also of configuration

Physical Chemistry
GeneralWhat is it that is rotated in a polarimetry experiment The priorities around asymmetrically substituted tetrahedral carbons The chiral molecules The chiral centers of molecules Plane polarized light The propagation plane of light

Physical Chemistry
General1 6 4 x 1024 2 7 2 x 1024 3 2 1 x 101 4 3 3 101 A photon in X region is more energetic than in the visible region X is 2 UV 3 Microwave 4 Radio wave 1 IR Electromagnetic radiations of wavelength 242 nm is just sufficient to ionise Sodium atom Then the ionisatio energy of Sodium in KJ mole is 1 494 65 2 400 3 247 4 600

Physical Chemistry
GeneralWhich of the following is not permissible arrangement of electrons is an atom A n 3 1 2 m 2 s 1 2 B n 4 1 0 m 0 s 1 2 C n 5 1 3 1 3 m 0 s 1 2 2 m 3 s 1 2 D n
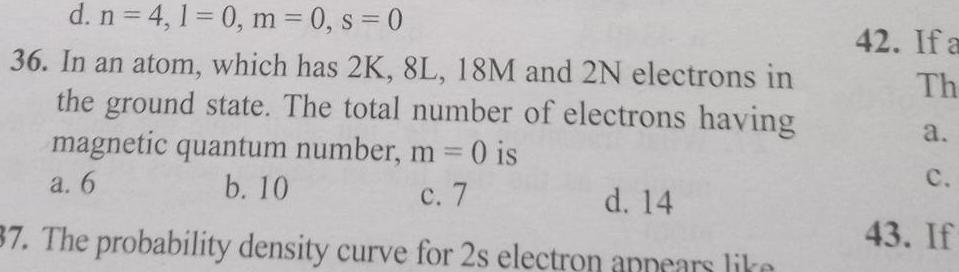
Physical Chemistry
Generald n 4 1 0 m 0 s 0 36 In an atom which has 2K 8L 18M and 2N electrons in the ground state The total number of electrons having magnetic quantum number m 0 is a 6 b 10 c 7 d 14 37 The probability density curve for 2s electron appears like 42 If a Th a C 43 If

Physical Chemistry
Generald 1s 7 Two simple harmonic motions A and B are giver respectively by the following equations y a sin cot TC y a sin oot Phase difference between the waves is a C EN R M TC 2 T 3r 6 3 b T 6 d Zero

Physical Chemistry
GeneralH3PO4 is a tribasic acid and one of its salt is NaH PO4 What volume of 1M NaOH solution should be added t 12 g NaH PO4 to convert it into Na3PO4 at wt of P 31 100 mL 200 mL CORRECT ANSWER 80 mL 300 ml

Physical Chemistry
General34 Suppose that a hypothetical H like atom gives a red green blue and violet line in spectrum Which jump according to figure would give off the violet spectral line n 4 a 3 1 c 4 1 n 3 n 2 n 1 b 2 1 d 3 2

Physical Chemistry
GeneralOne g of hydrogen is found to combine with 80 g of bromine One g of calcium valency 2 combines with 4 g of bromine The equivalent weight of calcium is 10 20 CORRECT ANSWER

Physical Chemistry
General12 A particle executes SHM its time period is 16 sec If it passes through the centre of oscillation then its velocity is 2 m s at time 2 sec The amplitude will be V

Physical Chemistry
General3 Series combin m 2 K 4 T 2n K K 20 When the displacement of a particle executing simple harmonic motion is half its amplitude the ratio of its kinetic energy to potential energy is a 1 3 b 2 1 c 3 1 d 1 2

Physical Chemistry
Generalb 0 69 m d 0 61 m c 0 41 m 14 A body executing SHM has velocity 10 cm s and 7 cm s when its displacements from the mean position are 3 cm and 4 cm respectively length of path a 10 cm b 4 8 cm c 4 cm d 11 36 cm J 2 KE 3 TE

Physical Chemistry
General2 Simple pendulum T 2x 32 The period of oscillation of a simple pendulum of constant length at surface of the earth is T Its time period inside mine will be a Increases c No change 33 If the b Decreases d None

Physical Chemistry
GeneralQuestion No 35 40 The equation zo RT is applicable to AO Multilayer film at surface BO Monolayer film at surface CO None of these DO Calculate osmotic pressure

Physical Chemistry
Generala 6 b 10 c 7 d 14 37 The probability density curve for 2s electron appears like R r th h r C a R2 R b R2 d r

Physical Chemistry
GeneralART Quantum theory of light and photoelectric Effect AIR service on Vividh Bharati is transmitted on 219 m band What is its transmission frequency in Hertz 1 1 3 x 106 Hz 4 6 5 x 106 Hz 2 1 9 x 106 Hz 3 1 x 106 Hz

Physical Chemistry
General23 The ratio of area covered in 2nd orbit to first orbit is d 4 1 a 1 1 b 16 1 c 1 16 24 If r be the radius of first Bohr s orbit of H atom the de Broglie s wavelength of an ola