General Questions and Answers

Physical Chemistry
GeneralThe oxidation reaction that takes place in lead storage battery during discharge is a Pb29 aq SO 2 aq b PbSO s 2H O 1 PbSO s PbO s 4H aq SO 20 aq 2e c Pb s SO2 aq d PbSO s 2e PbSO s 2e9 Pb s SO 20 aq

Physical Chemistry
General17 What would be percentage composition by volume of a mixture of CO and CH4 whose 10 5 mL requires 9 mL oxygen for complete combustion 1 CO 80 CH 20 3 CO 76 2 CH4 23 8 2 4 CO 90 CO 66 CH4 10 CHi 34

Physical Chemistry
General4 In an experiment it showed that 10 mL of 0 05 M solution of chloride required 10 mL of 0 1 M solution of AgNO3 which of the following will be the formula of the chloride X stands for the symbol of the element other than chlorine a X Cl c XC14 b XCl d X Cl Karnataka NEFT 2013

Physical Chemistry
GeneralA sample of KClO3 on decomposition yielded 224 mL of oxygen gas at NTP Calculate the weight of KCl produced 1 0 496 g 2 0 9937g 4 0 16g 3 1 986 g

Physical Chemistry
GeneralApproximate atomic weight of an element is 26 89 If its equivalent weight is 8 9 the exact atomic weight of element would be 1 26 89 3 17 8 2 8 9 4 26 7

Physical Chemistry
GeneralThe compound exhibiting maximum value of equivalent conductance in a fused state is 3 MgCl 4 BeCl SrCl 2 CaCl

Physical Chemistry
GeneralThe volume occupied by 7 23 1023 molecules of carbon dioxide and 3 01 x 1023 molecules of Argon at 0 C and 1 atm pressure is a 38 mL c 3 8 x 104 mL b 3 80 L d 3 8 x 103 mL

Physical Chemistry
GeneralExample 27 What is the temperature coefficient of the reaction if the rate grows 15 6 times when the temperature is increased by 30 K

Physical Chemistry
General1 1 point Round off 563 757 to four significant figures Write your answer with four sig figs in a standard number format and using scientific notati 563 757 standard format scientific notation

Physical Chemistry
General13 For an electron to have the same de Broglie wave length as that of a Deuteron its velocity should be times that of Deuteron 1 1836 2 1 1836 3 3672 4 1 3672

Physical Chemistry
General104 For the reaction H g 1 2O g H O l AC 7 63 Cal deg A H 5 68 3 kCal what H25 C will be the value in kCal of AH at 100 C 1 7 63 x 373 298 68 3 2 7 63 x 10 373 298 68 3 3 7 63 x 10 3 373 298 68 3 lots H 4 7 63 x 373 298 68 3

Physical Chemistry
GeneralThe specific conductance of saturated solution of silver chloride in pure water at 298 K is 1 26 x 10 6 ohm cm higher than that of water used The equivalent conductances at infinite dilution of AgNO3 HNO3 and HCl at this temperature are 132 8 421 2 and 426 9 ohm cm1 respectively Calculate the solubility of AgCl at 25 C Ans 0 001314 g litre

Physical Chemistry
GeneralConsider the following reaction sequence Sg s 80 g 8SO g 2SO g O g 2SO3 g How many grams of SO are produced from 1 mo Sg 1 1280 g 2 960 g 3 640 g

Physical Chemistry
GeneralMolarity of 29 W density is 1 22 g ml is 1 1 8 M 29 1000 X 98 14 75 2 3 6 M 3 2 4 M 4 1 2 M 29 98 H SO4 solution whose NCERT Pg 23 1 22 18 18000x10 122


Physical Chemistry
General4 Figure shows wave fronts in still water moving in the direction of the arrow towards the interface PQ between a shallow region and a deep denser region Which of the lines shown may represent one of the wave fronts in the deep region P shallow deep IV III II I Q

Physical Chemistry
GeneralQuestion A 0 141g sample of a phosphorus containing compound was digested in a mixture of HNO3 and H SO4 which resulted in formation of CO H O and H PO4 Addition of ammonium molybdate yielded a solid having the composition NH4 3 PO4 12 MoO3 The precipitate was filtered washed and dissolved in 50 0 mL of 0 20M NaOH 56 57 58 NH4 3PO4 12 MoO3 s 260H HPO 2 12M002 14H O 3NH g After boiling the solution to remove the NH3 the excess NaOH was titrated with 14 1 mL of 0 174M HCI The reacted milli moles of NaOH with solid having composition NH4 3PO4 12 MoO3 is A 7 55 B 5 75 C 6 65 D 8 25 Produced mole of NH3 is A 7 62 x 10 3 B 8 71 x 10 4 C 6 85 10 3 The percent of phosphorous in the given sample is B 5 56 A 3 36 C 6 38 D None of these D 7 25

Physical Chemistry
Generala In non reacting condition O Valency factor Total number of positive charge or negative charge present in the compou Solved Examples e 5 Na CO3 Fe SO4 3 FeSO 7H O

Physical Chemistry
Generala 26 93 J K D For a perfectly crystalline solid C p m molar entropy at 20 K is a 0 42 J K mol aT where a is constant If p m 1s 0 42 J K mol at 10 b 0 14 J K mol c 1 12 J K mol d zero

Physical Chemistry
GeneralWhat is the ApH final inital for 1 3 2 3 stages of neutralization of 0 1 M CH COOH with 0 1 M Na 1 2log 2 2 2 log 3 4 2 log 2 3 3 2 log 1 4

Physical Chemistry
GeneralHalf liter of perhedral releases V liters of oxygen at 1 atm and 273K The value of V 50

Physical Chemistry
Generalupto 2 10 electrons 4 6 electrons A d orbital can accomodate 1 5 electrons 3 2 electrons

Physical Chemistry
General49 What is the IUPAC name for the following compound a 5 methylcyclohexene 1 methyl 3 cyclohexene c CH3 b 4 methylcyclohexene d 1 methyl 4 cyclohexe

Physical Chemistry
GeneralIf a is the fraction of ammonia present by volume in an equilibrium mixture made from one volume of N and three volumes of H and P is the tota pressure then a c a 1 a a P a 1 a a P b d a 1 a a P a ap 1 a A

Physical Chemistry
General14 A giant molecule contains 0 25 of a metal whose atomic weight is 59 Its molecule contains one atom of that metal Its minimum molecular weight is 1 5900 2 23600 3 11800 4 100 59 0 4

Physical Chemistry
GeneralThe surface tension of which of the following liquid is maximum AIPMT Prelims 2005 1 H O 3 CH OH 2 CH 4 C H OH

Physical Chemistry
Generalmportance of the concept of enthalpy According to the first law of thermodynamics AU q w q PAV considering only P V work At constant volume AV 0 AU 9v Thus at constant volume heat absorbed or released qv by the system is equal to the change in internal energy of the system So we can determine the change in internal energy in a chemical reaction at constant volume by determining the amount of heat that is exchanged in the reaction However reactions are seldom carried out at constant volume as it is difficult to maintain constant volume during most reactions

Physical Chemistry
GeneralDetermine the percentage composition by mass of a mixture of anhydrous sodium carbonate and sodium bicarbonate from the following data wt of the mixture taken 2g Loss in weight on heating 0 11 gm

Physical Chemistry
General6 5 g of C H O is dissolved in 450 g of CH CN Nalorphene C H NO3 similar to morphine is used to combat withdrawal symptoms in narcotic users Dose of nalorphene generally given is 1 5 mg Calculate the mass of 1 5 10 m aqueous solution required for the above dose ring 350

Physical Chemistry
General2 O2 Na d H S 0 20 The passage of current through a solution of certain electrolyte results in the evolution of H g at cathode and Cl g at anode The electrolytic solution is 101000103 a Water b aq H SO4 aq NaClimut d aq CuCl2 When an 8

Physical Chemistry
GeneralAn aqueous solution of KIO3 was treated with excess of KI solution The solution is acidified with HCl The liberated 12 consumed 10ml of 1 0M thiosulphate solution to decolourise starch iodine complex Then millimoles of KIO3 consumed are

Physical Chemistry
General9 One of the lines in the spectrum of atomic hydrogen has wave number 533 16 cm What is the frequency of this line a 5 623 x 106 s 1 c 1 598x 10 3 S 1 b 1 876x 10 S 1 d 1 598x 10 4 S 1

Physical Chemistry
GeneralComment on the reactions of dihydrogen with i chlorine ii sodium and iii copper II oxide

Physical Chemistry
GeneralIf the boiling point of ethanol molecular weight 46 is 78 C the boiling point of diethyl ether molecular weight 74 is 1 100 C 2 78 C 3 86 C 4 34 C 13

Physical Chemistry
GeneralAmong following standard enthalpy of format non zero for 1 C graphite 3 H g 2 Br 1 4 12 g

Physical Chemistry
General5 When there can be more deviation in the behaviou of a gas from the ideal gas equation PV nRT 1 At high temperature and low pressure 2 At low temperature and high pressure 3 At high temperature and high pressure 4 At low temperature and low pressure 0

Physical Chemistry
GeneralCalculate the volume of O2 at 1 atm and 273 K required for the complete combustion of 2 64 L of acetylene C H at 1 atm and 273 K 2C H g 50 g 4CO2 g 2H 0 1 a 3 6 L b 1 056 L c 6 6 L d 10 L

Physical Chemistry
GeneralPROBLEM 48 A given sample of Na SO4 contains 6 02 1022 SO2 ions Calculate i number of Nations ii number of formula units and iii weight of the sample C00 1022 fomulo unite

Physical Chemistry
General2 Classification of Ele a Be and Mg are alkaline earth metal b K have larger radii than Ca c All d block elements are transition elements d He ion have larger size than F Incorrect statements is are 1 a c d 2 b c 3 a d

Physical Chemistry
GeneralA zero order reaction is one whose rate independent of 1 Temperature of the reaction 2 The concentration of the reactants The concentration of the products Activation energy

Physical Chemistry
GeneralIf 224 mL of a triatomic gas has a mass of 1g at 273 K and 1 atm pressure then the mass of one atom is 1 8 30 10 23 g 35 53 x 10 230 g 2 2 08 x 10 23 4 6 24 x 10 23 g

Physical Chemistry
General500 mL of a glucose solution contains 6 02 x 1022 molecules The concentration of the solution 1 54 www com

Physical Chemistry
Generaleryllium occurs naturally in the form of beryl The metal is produced from its ore lectrolysis after the ore has been converted to the oxide and then to the chloride How m rams of Be s is deposited from a BeCl solution by a current of 5 0 A that flows for 1 Atomic weight Be 9 a 0 840 b 1 68 c 1 42 d 1 08

Physical Chemistry
GeneralBy X ray diffraction it is found that nickel at mass 59 g mol crystallizes with ccp The edge length of the unit cell is 3 5 If density of Ni crystal is 9 0 g cm Then value of Avogadro s number from the data is a 6 05 x 1023 b 6 11 x 1023 c 6 02 x 1023 d 6 023 x 1023

Physical Chemistry
GeneralA B 2014 An aqueous solution of X is added slowly to an aqueous solution of Y as shown in List 1 The variation in conductivity of these reactions in List II Match List I with List II and select the correct answer using the code given below the lists List 1 S Codes C P C H5 3 N CH COOH X Y Q R KI 0 1M AgNO 0 01M X CH COOH KOH X NaOH HI X P 3 4 2 1 4 3 3 4 R2243 STTT 2 S 1 1 1 List II 1 Conductivity decreases and then increases 2 3 4 Conductivity decreases and then does not change much Conductivity increases and then does not change much Conductivity does not change much and then increases 2013
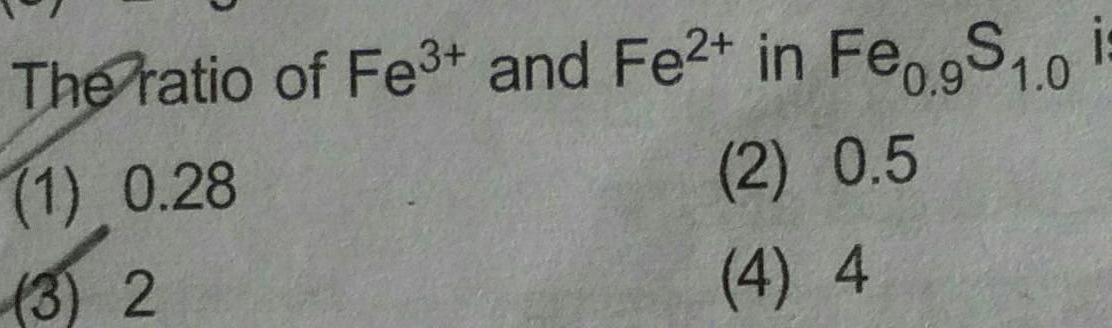

Physical Chemistry
General35 For every one 37Cl isotope there are three 35C isotopes in a sample of chlorine What will be the average atomic mass of chlorine a 35 b 37 c 35 5 d 35 6

Physical Chemistry
GeneralThe bonding in ammonium chloride a is covalent only b is electrovalent only c consists of three covalent nitrogen hydrogen bonds and an electrovalent bond between the ammonia molecules and the chlorine atom d consist of four covalent nitrogen hydrogen bonds and one electrovalent bond between the ammonium ion and chlorine atom

Physical Chemistry
General267 A 20 solution of KOH density 1 02g ml has molarity 3 64 A 1 33 B 3 64 C 3 31 D 4 41

Physical Chemistry
GeneralThe temperature rises by 18 F What is the rise on the Celsius scale ns 10 C