General Questions and Answers

Physical Chemistry
GeneralBasic strength of NH OH in presence of NH C 1 Increases 2 Remains unchanged 3 Decreases 4 Some times increases or sometimes decreases

Physical Chemistry
GeneralAmong the following processes identify those in which th change in internal energy AU is zero i isotherma compression of an ideal gas ii adiabatic expansion of a ideal gas iii free adiabatic expansion of an ideal go iv reversible cyclic process v irreversible cyclic process

Physical Chemistry
GeneralA sample was weighted using two different balances The results were i 3 929g ii 4 0 g How would the weight of the sample be reported a 3 93g c 3 9g b 3g d 3 929g

Physical Chemistry
Generalat A 20 litre container at 400 K contains CO2 g a pressure 0 4 atm and an excess of SrO neglect the volume of solid SrO The volume of the container is now decreased by moving the movable piston fitted in the container The maximum volume of the container when pressure of CO2 attains its maximum value will be Given that SrCO3 s SrO s Kp 1 6atm 1 10 litre 3 2 litre 2 4 litre 4 5 litre CO g

Physical Chemistry
GeneralAt 518 C the rate of decomposition of a sample of gaseous acetaldehyde initially at a pressure 363 Torr was 1 00 Torr s when 5 had reacted and 0 5 Torr s when 33 had reacted The order of the reaction is 1 O 3 3 2 2 4 1 MAINS 2018

Physical Chemistry
GeneralFor the reaction PC13 g Cl g of equilibrium can be shifted to the right by a Increasing the temperature b Doubling the volume PCI g the position c Addition of Cl at constant volume d Addition of equimolar quantities of PCI and PC1

Physical Chemistry
General4 A 200 g sample of hard water is passed through the column of cation exchange resin in which H is exchanged by Ca The outlet water of column required 50 mL of 0 1M NaOH for complete neutralization What is the hardness of Ca ion in ppm A 250 ppm B 500 ppmC 750 ppmD 1000 ppm

Physical Chemistry
GeneralFor a reversible reaction 2NO g O g 2NO g the rate expression is given as 2 6x10 NO 0 4 1 NO The rate constant of forward reaction will be dt net dx A 1 58 10 3 X C 10 66 10 B 2 6 10 3 D 1 06 10

Physical Chemistry
GeneralCalculate H of 0 01 M A B salt solution if K HB 10 2 a A 10 M B 10 7 M C 6 2 x 10 3 D 1 6 x 10 12 M PHA 11 12

Physical Chemistry
Generalii Suppose the human population of the world is 3 x 1010 If 100 molecules of sugar C12H22011 are distributed per head what is the total quantity of sugar required for distribution

Physical Chemistry
GeneralExample 17 Calcium phosphide Ca P formed by reacting calcium orthophosphate Ca3 PO4 2 with magnesium was hydrolysed by water The evolved phosphine PH3 was burnt in air to yield phosphorus pentoxide P O5 How many grams of magnesium metaphosphate would be obtained if 19 2 g of magnesium were used for reducing calcium phosphate At wt Mg 24 P 31 Ca3 PO4 2 Mg Ca3P MgO Ca3P H O Ca OH PH PH O P O5 H O MgO P O5 Mg PO3 2 Magnesium metaphosphate
Physical Chemistry
GeneralA beaker containing 18 g of glucose in 100g H O and another containing 18 g of urea in 100g H O places under a bell jar and air is removed After a course of time when equillibrium reaches how much water will be transferred from one beaker to the other A 20g 0 B 25g8 2 C 30g 2 A D 50g

Physical Chemistry
GeneralThe volume of a gas in discharge tube is 1 12 x 107 mL at STP Then the number of molecule of gas in the tube is 1 3 01 x 104 3 3 01 x 10 2 2 3 01 x 1015 X 4 3 01 x 1016

Physical Chemistry
GeneralIn which of the following compounds a exhibits two different oxidation states b NH NO a NH OH d N H c N H at of the following arrang an elemen

Physical Chemistry
GeneralMole C A sample of ammonium phosphate NH4 3PO4 contains 3 18 moles of hydrogen atoms The number of moles of oxygen atoms in the sample is 55151 1 0 265 3 1 06 2 0 795 4 4 00

Physical Chemistry
GeneralThe electron gain enthalpy Heg of an element A is 200 kJ mol The electron affinity A of A a absolute temperature can be 200 kJ mol 2 201 kJ mol 4 100 kJ mol 3 200 kJ mol
Physical Chemistry
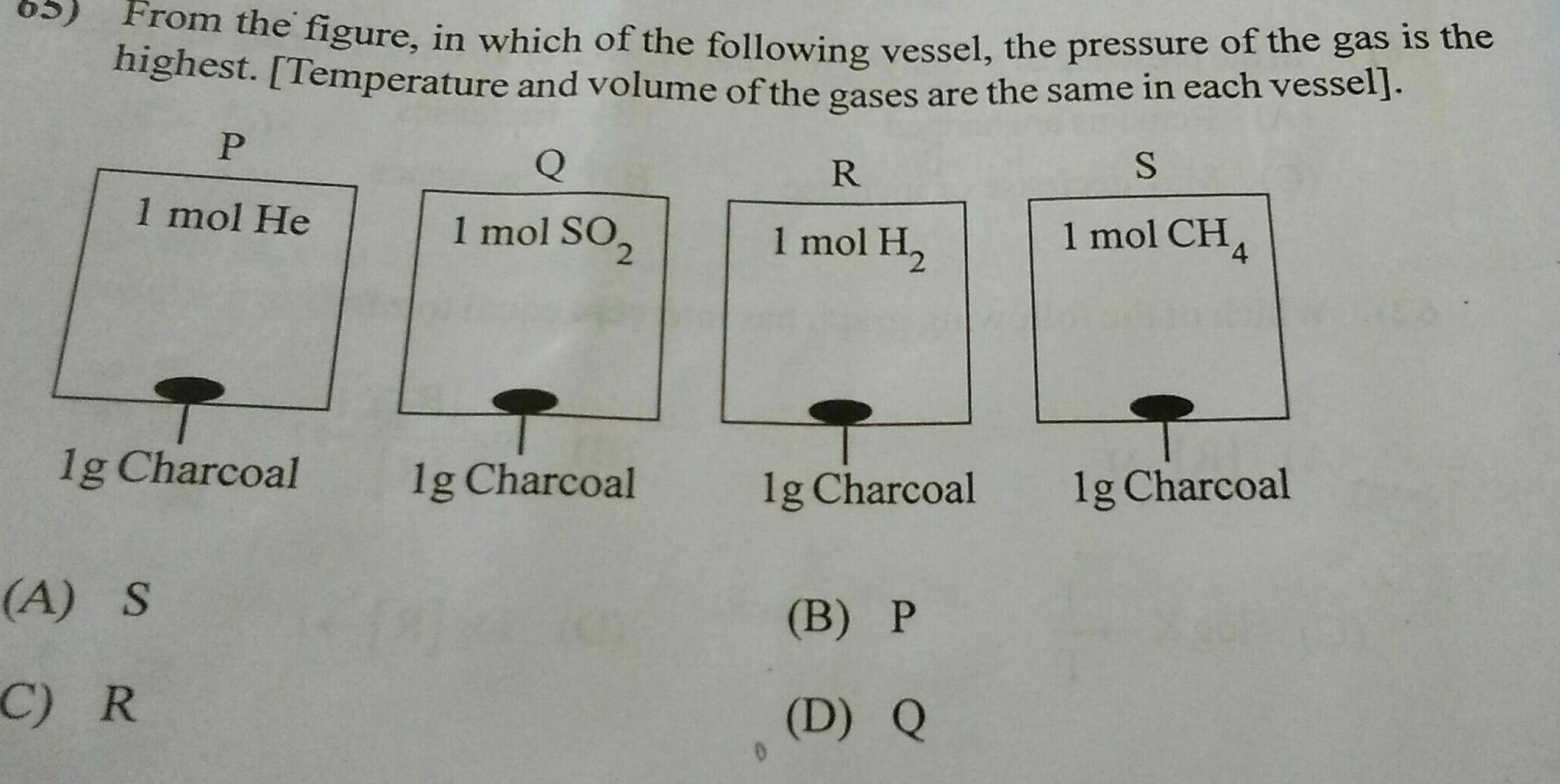
GeneralFrom the figure in which of the following vessel the pressure of the gas is the highest Temperature and volume of the gases are the same in each vessel S P 1 mol CH4 1 mol He lg Charcoal A S C R Q 1 mol SO 2 lg Charcoal R 1 mol H lg Charcoal B P D Q lg Charcoal

Physical Chemistry
General00 One litre of a mixture containing BaF and H SO4 was taken for analysis 25 mL of this mixture was treated with 20 0 mL of 0 1 NKOH for complete neutralisation Another 25 mL of the mixture was added to 100 mL of 0 05 N K CO3 solution and precipitate was filtered off The filtrate required 12 mL of 0 025 M oxalic acid solution using phenolphthalein as indicator Find the strength of BaF and H SO4 in mixture Ans BaF 6 372 g L H SO4 3 92 g L

Physical Chemistry
GeneralDecomposition of A g follows first order kinetics according to the reaction A g 2B g C g If initial pressure of A g is 10 atm and total pressure after 70 sec is 20 atm then In2 0 7 A Rate constant of reaction is 0 01 sec B Partial pressure of B at 70 sec is 10 atm C Partial pressure of C at 140 sec is 7 5 atm D Initial rate of reaction is 0 1 atm sec 13

Physical Chemistry
GeneralThe number of electrons in 1 6 g of CHA approximately 1 25 x 1024 3 6 1023 2 1 5 1024 4 3 0 1024

Physical Chemistry
Generalper second is xx 10 0 Find the value of x 6x10 305 0 x24x365 Rahul Dravid wants to wear 6 023 1021 Ag atoms in the form of a ring His Silver Gold Copper alloy ring consists of 20 of Silver The mass of the ring is 0 9x What is x both the sides

Physical Chemistry
General25 ml of aqueous solution of BaCl required 20 mol of 1M Ag NO when titrated using K CrO indicator Assuming 100 ionisation the elevation of boiling paint BaC1 solution is K H O 0 52 K kg mol 1 0 21 K 2 0 42 K 3 0 62 K 974 0 72

Physical Chemistry
GeneralWhich contains greater number of oxygen atoms 1 1 gm of O 2 1 gm of O 3 1 gm of 03 4 All have same number of oxygen atoms

Physical Chemistry
GeneralAn intimate mixture of ferric oxide Fe O3 and aluminium Al is used in solid fuel rockets Calculate the fuel value per gram and fuel value per cc of the mixture Heats of formation and densities are as follows H Al2O3 399 Kcal mol and Hr Fe O3 199 Kcal mol Density of Fe O3 5 2 g cc Density of Al 2 7 g cc 1988

Physical Chemistry
Generaland F are 72 pm and 136 pm respectively then choose 5 Atomic radius and ionic radius of F the CORRECT statement s A Ratio of size in term of volume 6 75 B increase in size in term of volume 5 75 in formation of F to F C Ratio of size in term of volume 675 du D increase in size in term of volume 575

Physical Chemistry
GeneralIn a compound Carbon is 52 2 Hydrogen is 13 Oxygen is 34 8 are present and molecular mass of the compound is 92 Calculate molecular formula of the compound

Physical Chemistry
Generaltion 1 If the mass of neutrons is doubled mass of electron is halved then find out the new atom of C12 and the percent by which it is increased Step 1 C 2 e 6 1 p 6 6 amu n 6 6 amu 12 amu If the mass of neutrons is doubled and mass of er is halved then n 12 amu 1 Increment 18 amu p 6 amu Note mass of er is negligible so it is not considered in atomic mass Final mass Initial mass Initial mass Step 2 100 18 12 12 100 50

Physical Chemistry
GeneralV2 V2 Pav RT d nRT dv P nRT V V V As the process is isothermal so Tremains constant Wrev Wrev nRT 1 V 2 j dv V1 TZ

Physical Chemistry
GeneralWhen same quantity of electricity is passed the electrochemical equivalents of the two metals liberated at the electrodes have the same ratio as that of their a atomic masses c equivalent masses b molecular masses d any of the three

Physical Chemistry
Generalthe iii A compound on analys is shows C 40 H 6 67 and 0 53 33 Determine empirical formula of the compound If molar mass of the compound is 30g mol 1 what is its molecular mass 1 1

Physical Chemistry
General49 A gaseous hydrocarbon gives upon combustion 0 72 g of water and 3 08 g of CO2 The empirical formula of the hydrocarbon is

Physical Chemistry
GeneralMolarity of NaOH in 200 mL of an aqueous solution of it is 1M find the change in molarity if 2g of NaOH is added to it 1 1 25 M 3 0 50 M 2 0 25 M 4 0 75 M

Physical Chemistry
GeneralThe volume and temperature of 2 mol of an ideal gas are 10L 27 C respectively The gas is allowed to expand in an isothermal reversible process to attain final volume of 25 L Calculate the maximum work a done Ans We know that maximum work is obtained in a reversible isothermal expansion Now work done by an ideal gas in an isothermal V reversible expansion w nRT In V Given n 2 T 273 27 300K V 10 L V 25L w 2x 8 314 x 300 In 25 J 4570 82 J 10 The maximum work done by the gas 4570 82 J

Physical Chemistry
General1 Choose the most correct option A A sample of pure water whatever the source always contains by mass of oxygen and 11 1 by mass of hydrogen a 88 8 b 18 c 80 d 16 B Which of the following compounds can NOT demonstrate the law of multiple proportions a NO NO c H O H O C Which of the following temperature will read the same value on celsius and Fahrenheit scales a 40 b CO CO d Na S NaF b 40 c 80 d 20 D SI unit of the quantity electric current is a Volt c Candela E In the reaction N 3H 2NH the ratio by volume of N H and NH is 1 3 2 This illustrates the law of b Ampere d Newton a definite proportion b reciprocal proportion Exercises c multiple proportion d gaseous volumes a 4 5 c 0 25 F Which of the following has maximum number of molecules a 7 g N c 8 g 0 G How many g of H O are present in 0 25 mol of it b 2 g H d 20 g NO b 18 d 5 4 H The number of molecules in 22 4 cm of nitrogen gas at STP is a 6 022 x 1020 b 6 022 x 1023 c 22 4 x 1020 d 22 4 x 1023 I Which of the following has the largest number of atoms a 1g Au s b 1g Na s 2 Answer the following questions A State and explain Avogadro s B Point out the difference betwe carbon and 12 u of carbon C How many grams does an hydrogen weigh D Calculate the molecular ma following in u a NH b CH COOH c CH E How many particles are pre mole of a substance F What is the SI unit of amc substance G What is meant by molar vol gas H State and explain the law of com of mass I State the law of multiple propc 3 Give one example of each A homogeneous mixture B heterogeneous mixture C element 4 Solve problems D compound A What is the ratio of molecules of NH and 1 mole of HNO A B Calculate number of moles of E in 0 448 litre of hydrogen gas c Ans 0 C The mass of an atom of hyd 1 008 u What is the mass of 1 of hydrogen 18 144 u D Calculate the number of atom of the following Given Atom of I 127 u a 254 u of iodine I b 254 g of iodine I Ans 2 atoms 1 2044 x 10 E A student used a carbon pencil his homework The mass of th found to be 5 mg With the help calculate The

Physical Chemistry
General7 What will be the pH of the solution if 0 01 moles of HCl is dissolved in a buffer solution containing 0 02 moles of propanoic acid K 1 34 x 10 5 and 0 0152 moles of salt at 25 log 0 173 1 3 11 0 76 2 4 11 3 5 11 4 6 11

Physical Chemistry
General2 g of base X requires 100 ml of 0 4 N acid fo complete neutralisation The equivalent weight c base is 1 50 3 5 2 100 4 150

Physical Chemistry
GeneralB 228 5 kJ mol C 114 25 kJ mol 1 D 114 25 kJ mol 2 31 Enthalpy of neutralization of H PO acid is 106 68 kJ mol using NaOH If enthalpy of neutralization of HCI by NaOH is 55 84 kJ mol Calculate AHjonization of H3PO3 into its ions A 50 84 kJ mol B 5 kJ mol C 2 5 kJ mol D 60 84 kJ mol 32 The lattice enthalnu of LLO

Physical Chemistry
GeneralWhat is mass of sodium acetate CH3COONa required to make 200 ml of 0 245 molar aqueous solution Molar mass of CH3COONa 82 g mol NCERT Pg 23 1 3 g 3 5 0 2 4g 4 6 q

Physical Chemistry
GeneralThe following sequence Atomic weight of Zn 2ZnS 30 2ZnO 2SO ZnO C Zn CO of reactions may be 65 S 32 How many tons of Zn can be obtained form 32 33 tons of ZnS assuming that the yield is 75 for each reaction A 5 B 100 C 16 2 D 16 4

Physical Chemistry
GeneralA 25 watt bulb emits monochromatic yellow light of wavelength of 0 57 m Calculate the rate of emission of quanta per second

Physical Chemistry
GeneralA short solenoid of radius a number of turns per unit length n and length L is kept coaxially inside a very long solenoid of radius b number of turns per unit length 1 What is the mutual inductance of the system a Monb n n L c Mona n n L b Mona n n L d Mob n n L

Physical Chemistry
GeneralWhat volume of 6 0 M H SO4 should be mixed with 10 L of 0 1 M H SO4 to make 20 0 L of 3 0 M H SO4 upor dilution to volume a 1 7 L c 8 3 L b 5 L d 10 0 L

Physical Chemistry
GeneralQ 28 Assuming no change in volume calculate the minimum mass of NaCl necessary to dissolve 0 010 m AgCl in 100 L solution S 0 01 10 0 K AgCl 3 105 K AgCl 1 10 1 Ag Cs1 Ag 5 4 Ag 201 San B 2015 NO C 2x15 N Ag U X S C

Physical Chemistry
General2 108 atoms of carbon are arranged side by side Calculate the radius of carbon atom if the length of this arrangement is 2 4 cm

Physical Chemistry
Generalv 0 341 x 10 2 34 1 x 1010 or v 5 84 x 105 m s Q 2 10 Electromagnetic radiation of wavelength 242 nm is just sufficient to ionize the sodium atom Calculate the ionization energy of sodium in kJ mol or

Physical Chemistry
General1 1 M 8 0 3 M If an ideal solution is made by mixing 2 moles of benzene p 266 mm Hg and 3 moles of another liquid 2 236 mm Hg The total vapour pressure of the solution at the same temperature would be 4 3 1 502 mm Hg 3 600 mm Hg 00 2 248 mm Hg 4 250 6 mm Hg ml of liquid A and 25 ml of liquid B is mixed to give a solution which does not obey Raoult s law The olution 21 Can be or than 125 ml

Physical Chemistry
Generalg mixture of CaCO3 and NaCl reacts completely with 100 ml of HCI The of CaCO in the N 10 mixture is 1 40 500 2 60 4 80

Physical Chemistry
GeneralWhich of the following salts is the most basic in aqueous solution 1 Pb CH3COO 2 3 CH3COOK 2 AI CN 3 4 FeCl3 IS HE

Physical Chemistry
GeneralWhich of the following will decrease with dilution at a given temperature a pH of 10 3 M acetic acid solution b pH of 10 3 M aniline solution c degree of dissociation of 10 3 M acetic acid d degree of dissociation of 10 3 M aniline solution 64 Na MNII CLIf 10 mL of 0 001 M HC

Physical Chemistry
GeneralA sample of 4CO was mixed with ordinary 2CO for studying a biological tracer experiment The 10 mL of this mixture at STP possess the rate of 104 disintegration per minute How many milli curie of radioactive carbon is needed to prepare 60 litre of such a mixture