General Questions and Answers

Physical Chemistry
Generald is not 161 Consider the argon atom For how many electrons does this atom have m 17 d 2 a 1 b 6 c 4

Physical Chemistry
GeneralTry Yourself 1 Conductivity of 0 12 M CUSO solution at 298 K is 1 8 x 10 2 S cm 1 Calculate its equivale conductivity

Physical Chemistry
GeneralFor a given exothermic reaction K and K are the equilibrium constants at temperatures T and T2 respectively Assuming that heat of reaction is constant in temperature range between T and T it is readily observed that 1 2 1 K K p 3 K K 2 Kp Kp 1 4 K

Physical Chemistry
Generalx100 100 50 10 Weight of N CO3 0 9031 g of a mixture of NaCl and KCI on treatment with H SO4 gave 1 0784 g of a m of Na SO4 and K SO4 Calculate percentage composition of the original mixture

Physical Chemistry
GeneralAn open vessel containing air is heated from 300K to 400K The fraction of air originally present which goes out of it is a 3 4 c 2 3 b 1 4 d 1 8

Physical Chemistry
GeneralA solution of palmitic acid in benzene contains 4 24g of acid per litre When this solution is dropped on water surface benzene gets evaporated and palmitic acid forms a unimolecular film on surface If we wish to cover an area of 500 cm with unimolecular film what volume of solution should be used The area covered by one palmitic acid molecule may be taken as 021 nm2 Mol wt of palmitic acid is 256 2

Physical Chemistry
General4 A sample supposed to be pure CaCO3 is used to standardise a solution of HCl The substance really was a mixture of MgCO3 and BaCO3 but the standardisation of HCl was accurate Find the percentage of BaCO3 and MgCO3 in mixture

Physical Chemistry
General13 An electron beam can undergo diffraction by crystals Through what potential should a beam of electrons be accelerated so that its wavelength is equal to 1 6 1 58 90 V 3 45 35 V 2 85 75 V 4 105 31 V

Physical Chemistry
General3 Which of the following reactions defines AH a C diamond O g CO g 640 to gian 1 b H g F g HF g wollon od no ed mot to tood c N g 3H g 2NH3 g Lo all aure tolygos to noted to rese d CO g 0 g CO g

Physical Chemistry
General4 Zn rod is placed in 100 mL of 1M CuSO solution so that molarity of Cu2 changes to 0 7 M The molarity of SO2 at this stage will be 4 1 0 8 M 2 1 M 3 0 7 M 4 1 8 M

Physical Chemistry
GeneralA solution of 500 mL of 0 2 M KOH and 500 mL of 0 2 M HCl is mixed and stirred the rise in temperature is T The experiment is repeated using 250 mL each of solution the temperature rise is T Which of the following is true a T T T b T 2T c T 4T d T 9T sidient

Physical Chemistry
General26 The absolute configuration of the following compound is H CI a 2 S 3 R c 2 B 35 CH 2 Cl H 3 C H b 2S 3S d 2 R 3 R 2003
Physical Chemistry
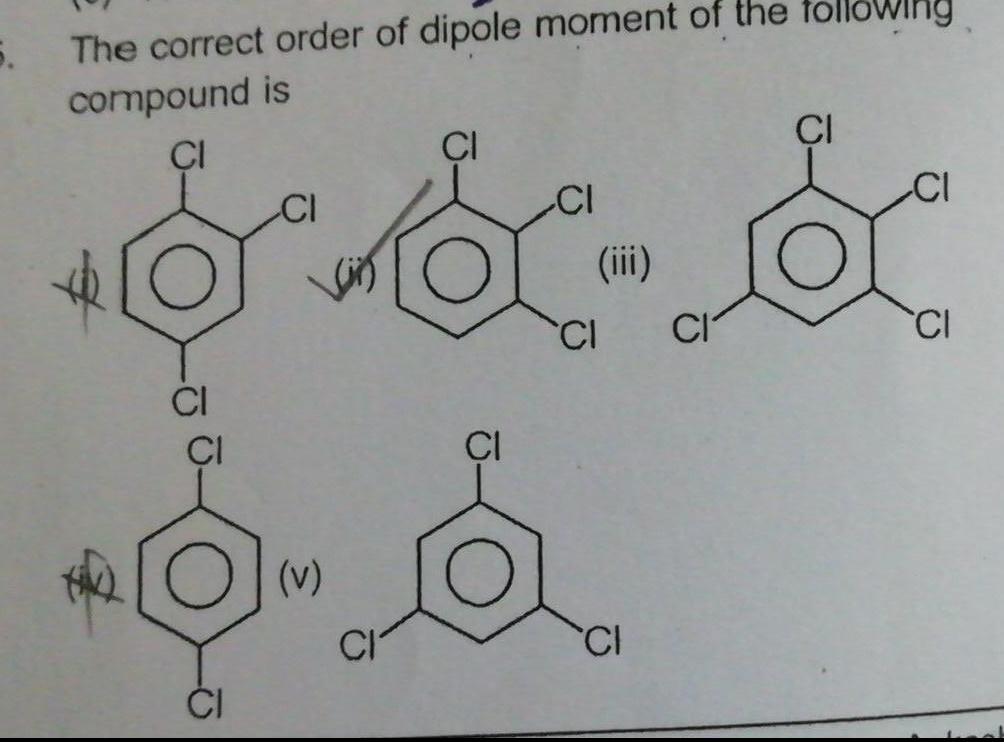
General5 The correct order of dipole moment of the compound is CI CI CI v CI CI iii CI CI CI owing CI

Physical Chemistry
GeneralWhich of the following is most likely formula of platinum complex if of total chlorine 4 of the compound is precipitated by adding AgNO to its aqueous solution 1 PtCl 6H 0 2 PtCl4 5H O 3 PtCl 2H O 4 PtCl 3

Physical Chemistry
General44 gram sample of a natural gas consisting of methane CH and ethylene C H was burned in excess of oxygen yielding 132 gm CO and some H O as products What is the mole of ethylene in the sample

Physical Chemistry
GeneralE A student used a carbon pencil to write his homework The mass of this was found to be 5 mg With the help of this calculate a The number of moles of carbon in his

Physical Chemistry
General586 Which of the following concentration of metals in drinking water is not prescribed 1 Fe 0 2 ppm 2 Cu3 0 ppm 5 3 Cd 2 0 ppm 4 Zn 5 0 ppm 880

Physical Chemistry
GeneralD Add the suffixes en ful less y ly tal able al ive or ous to make adjectives You might get more than one adjective from some words Watch your spellings NOUNS gold wood 3 silk 4 skill 5 care 6 cheer 7 power 8 coward 9 love 10 live 11 friend 12 remark 13 measure 14 response 15 appreciate ADJECTIVES golden silhey skillly Lovely the livey Sriendly desporbely abbrevilaty NOUNS 16 attract 17 elude 18 taste 19 storm 20 star 21 sleep 22 cream 23 powder 24 length 25 hand 26 bulb 27 ridicule 28 horizon 29 element 30 region ADJECTIVES part starry Speeb cream powse longth hand bulls ridicale 0001201 element TIO

Physical Chemistry
GeneralUsing the following Latimer diagram for bromine 7 pH 0 BrO4 1 82 V Bro 1 50 V 7 8 HBrO 1 595 V 1 06552 V Br the species undergoing disproportionation is a BrO4 b BrO3 c HBrO d Br Br

Physical Chemistry
General500 ml solution of 1M CaBr solution d 1 2 gm ml is mixed with 500 ml of 1m Nal solutio d 1 15 gm ml Choose the correct statements regarding resulting solution At wt of Br 80 I 127 Ca 40 Molarity of CaBr in resulting solution is 0 5M BMolality of Nal in resulting solution is 0 5M Volume of resulting solution is 1000 ml D Density of resulting solution is 1000 kg m

Physical Chemistry
General4 To 50 litre of 0 2N NaOH 5 litre of 1N HCl and 15 litre of 0 1N FeCl3 solution are added What weight of Fe 03 can be obtained from the precipitate Also report the normality of NaOH left in the resultant solution

Physical Chemistry
Generalc 5x10 kg 5 The number of atoms in 4 25 g NH3 is approximately b 1 5x 1023 roles as d 6 1023 orn atomic weight scale is based on a 1x 1023 c 2 1023

Physical Chemistry
General2 The wavelength of the first line of the He ion spectral series whose interval between the extreme lines is 2 725 x 106 m is R 1 09 107 m a 471 82 nm c 1019 37 nm b 4718 2 nm d 165 14 nm

Physical Chemistry
General3 100 mL of O gas diffuses in 10 s 100 mL of gas X diffuses in t sec Gas X and time t can be 1 H 2 5 s 2 SO2 16 s 3 CO 10 s 4 He 4 s

Physical Chemistry
GeneralAL 2017 QUILIBRIUM 3 PHYSICAL CHEMISTRY Reaction N O NO NO was studied by taking 2 mol L of N O initially A equilibrium degree of dissociation of N O was found to be 10 What is the ratio of equilibrium concentration of N O NO and NO 1 19 1 1 3 1 1 9 The equilibrium H g CO g 2 9 1 1 4 18 1 1 constant for reaction H O g CO g is 1 8 at 1000 C If one mole of H and 1 0 mole of CO are placed in a one litre flask The final

Physical Chemistry
GeneralA particle executes SHM of amplitude A i At what distance from the mean position is its kineti energy equal to potential energy ii At what points is its speed half the maximum speed

Physical Chemistry
GeneralThe concentration of H and concentration of OH of a 0 1 aqueous solution of 2 ionised weak acid is Ionic product of water 1 10 4 CBSE PMT 1999 DPMT 2004 a 2x10 M and 5x10 M b 1x10 M and 3 10 M c 0 02x10 M and 5 10 11 M d 3x10 2 M and 4x10 13 M

Physical Chemistry
General31 12 grams of a mixture of sand and calcium carbonate on strong heating produced 7 6 grams of residue How many grams of sand is present in the mixture SERIES for Sri Chaitanya 23

Physical Chemistry
GeneralA 10 cm air column is trapped inside the tube having uniform area of cross section by 6 cm of Hg and is placed horizontally to the plane Calculate the length of air column when the tube is placed vertically with opened end up 1 9 26 cm 2 10 cm 3 10 25 cm 4 11 5 cm

Physical Chemistry
General5 An organic compound on analysis gave C 54 2 H 9 2 by mass Its empirical formula is a CHO CH 0 c C H O C H O The relative number of atoms of elements X and Y in b d

Physical Chemistry
GeneralD Volume of a tyre 10 litre when inflated The tyre is inflated to a pressure of 3 atm at 17 C with air Due to driving the temperature of tyre increases to 47 C a What would be the pressure at this temperature b How many litre of air measured at 47 C and pressure of 1 atm should be let out to restore the tyre of 3 atm at 47 C

Physical Chemistry
General7 Formation of ethane from calcium carbide takes place as follows CaC 2H O Ca OH C H C H 2H C H6 The amount of ethane gas obtained from 72 kg of water is 1 60 kg 3 40 kg 2 20 kg 4 52 kg

Physical Chemistry
General8 A completely filled d orbital d 0 is of a spherical symmetry b octahedral symmetry c tetrahedral symmetry d unsymmetry

Physical Chemistry
General1 10 mL of 1 mM surfactant solution forms a monolayer 2 covering 0 24 cm on a polar substrate If the polar head is approximated as a cube what is its edge length 2019 Main 9 April II d 2 0 nm a 2 0 pm b 0 1 nm c 1 0 pm

Physical Chemistry
GeneralA B C and D are four metals A can displace D from its salt solution but B and C cannot displace D B can displace hydrogen H for a dilute solution of a mineral acid but C cannot The reduction potentials of A B C D and H i e hydrogen are in the order A C H B D A B A B C D H D H A C D B C A D B H C

Physical Chemistry
GeneralIn the intramolecular Cannizzaro s reaction when glyoxal is reacted with concentrated NaOH the n factor of glyoxal is 1 4 3 2 2 3 4 1

Physical Chemistry
GeneralWhen SO is passed through the solution of potassium iodate the oxidation state of lodine changes from 1 5 to 0 3 5 to 0 2 5 to 1 7 to 1

Physical Chemistry
Generala IIT 2001 4 A graph is plotted between PV along Y axis and P along X axis where Vm is the molar volume of a real gas Find the intercept along Y axis IIT 2004

Physical Chemistry
GeneralOne or more than one correct option 11 The following is are endothermic reaction s a Combustion of methane b Decomposition of water c Dehydrogenation of ethane to ethylene d Conversion of graphite to diamond

Physical Chemistry
General578 The principle chromatography is 1 Adsorption 3 Solubility involved min in paper 2 Partition 4 Volatility

Physical Chemistry
GeneralHow many moles of KMnO are reduced by 1 mole of ferrous oxalate in acidic medium 76 How 1 1 2 5 3 HOS 1 3 3 4 3 GH

Physical Chemistry
General28 How much water is added to 100 ml 0 5M H SO4 solution so that its molarity becomes 0 1M 1 500 ml 2 400 ml 3 100 ml 4 200 ml



Physical Chemistry
General1 The density of helium is 0 1784 kg m at STP If a given mass of helium at STP is allowed to expand to 1 400 times of its initial volume by changing P and T compute its resultant density

Physical Chemistry
General28 What is the molarity and molality of a 13 solution by weight of sulphuric acid with a density of 1 02 g mL To what volume should 100 mL of this solution be diluted in order to prepare a 1 5 N solution le 1978 2M HO a 0 027 mmHg b 0 031 mmHg c 0 017 mmHg d nac 0001 bulib 3M

Physical Chemistry
General84 A drop 0 05 mL of 12 M HCI is spread over a thin sheet of aluminium foil thickness 1x10 cm and density 2 7 gmL Assuming whole of the HCI is used to dissolve Al The maximum area of hole produced in the foil is 1 2x10 cm 2 2 20 10 cm 3 200 10 cm 4 2000 10 cm

Physical Chemistry
GeneralIn hydrogen atom the de Broglie wavelength of an electron in the second Bohr orbit is Given that Bohr radius a 52 9 pm a 211 6 pm b 211 6 pm c 52 9 pm d 105 8 pm Odisha NEET 2019

Physical Chemistry
GeneralNGLE CORRECT How many hydrogen atoms are present in 0 1 mole of mohr s salt FeSO4 NH4 2 SO4 6H O A 10 NA X B 14 NA C 2 NA D None of these of uptor at room temperature is 1 g ml How many molecules are there in a drop of water if i

Physical Chemistry
GeneralOne litre solution contains 1M HOCI K 10 and 1 M NaOH What is the pH of th solution 1 8 3 5 4 2 2 11