General Questions and Answers

Physical Chemistry
General66 Which of the following has minimum flocculation value for positively charged sol 2 A CI C PO 2 b SO d Fe CN 6

Physical Chemistry
General30 Non metals are usually poor conductors of heat and electricity They are non lustrous non sonorous non malleable and are coloured a Name a lustrous non metal V b Nam b Name a non metal which exists as a liquid at room temperature c The allotropic form of a non metal is a good conductor of electricity Name the allotrope d Name a non metal which is known to form the largest number of Va compounds e Name a non metal other than carbon which shows allotropy Name a non metal which is required for combustion

Physical Chemistry
GeneralAt 527 C the reaction given below has K 4 NH3 g 1 3 N 9 H 9 2 g 2 What is the K for the reaction p N g 3H g 2NH3 g 1 16 800 R 3 2 2 800R 4 2 4 None of these

Physical Chemistry
Generaleventh period This period corresponds to filling of electrons in the seventh energy level n 7 Like sixth period it is expected to accommodate 32 elements corresponding to filling of 16 orbitals one 7s seven 5f five 6d and three 7p However at present this period is incomplete consisting of 28 elements Last element of this period will have an atomic number of 118 and it will occupy its position in the inert gas family

Physical Chemistry
General1 Objective type questions A Tick the correct answer 1 Which of the following does not belong to Rabi crop a wheat b paddy c legumes 2 Which can be used for both harvesting and threshing a sickle c combine b drills 3 Which helps in turning and loosening of soil a earthworm b insects 4 Compost includes a vegetable wastes b fruit peels 5 Weeds can be removed by a pesticides b weedicides D Match the following 1 Wheat 2 Maize 3 Furrow 4 Weedicides 1 Soil provides essential 2 3 4 Implement used to turn over and break up the soil is called 5 The removal of unwanted plants from the field is called 6 The sowing of seeds manually is called 7 Bee keeping is called 8 Growing of crops on rotation basis in a field is called 9 On large scale grains should be stored in c flies 1 Excessive irrigation to the crop reduces air in the soil spaces 2 Manure is decayed organic material 3 Chemical fertilisers are highly insoluble in water 4 Nitrates are the soluble salts of nitric acid 5 Repeated crop production in a soil increases its nitrogen content C Fill in the blanks 1 Chemicals II Kharif crop Ravi crop III IV Irrigation c animal dung c nitrites B Read the following statements and write True or False against them I Differentiate the following 1 Rabi and Kharif Crops emport and Green Manure d none of these to the crop fertilisers increase the nitrate content and alkalinity of the soil is an organism which damages the crop d winnow d bees and d all of these d none of these 2 Ploughing and Labelling 4 Sowing and Winnowing

Physical Chemistry
General13 Bredig arc method cannot be used to prepare colloidal solution of which of the following a Pt b Fe c Ag d Au 4 Which one is true statement

Physical Chemistry
GeneralThe range of most suitable indicator which should be used for titration of X Nat 0 1M 10 ml with 0 1 M HCI should be Given kb x 10 6 B 3 5 A 2 3 C 6 8 Read the follwing statements I II carefully and select write option D 8 10

Physical Chemistry
General143 A cell constant is generally found by measuring the solution of aqueous solution of unni b b KC1 abam tasbure A d MgCl Prosodal conductivity of aqueous a BaCl pado gn c NaCl 065

Physical Chemistry
General1000 g aqueous solution of CaCO contains 10 g of calcium carbonate concentration of the solution is 1 10 ppm 3 1000 ppm 2 100 ppm 4 10 000 ppm rib sin

Physical Chemistry
GeneralIn two different polar molecules the ionic charge is 9 6 x 10 10 e s u and 3 2 x 10 19 coulombs respectively If inter ionic distance in both the molecules is 1A unit then the dipole moment are respectively 1 4 8 D 4 8 D 3 9 6 D 96D 2 1 D 4 8 D 4 3 33 D 4 8 D

Physical Chemistry
GeneralFor real gases the relation between P Vand Tis given by van der Waals equation P an V nb nRT where a and b are van der Waals constants nb is approximately equal to the total volume 1 of the molecules of a gas a is the measure of magnitude of intermolecular attraction i Arrange the following gases in the increasing order of b Give reason O CO2 H2 He i Arrange the following gases in the decreasing order of magnitude of a Give reason CH4 O H2

Physical Chemistry
GeneralEqual amounts of solutions of NaOH having concentration 10 wt wt and 20 wt wt are mixed What will be molality of the NaOH in the resulting solution 1 3 0 m 3 15 m 2 0 15 m

Physical Chemistry
Generala How Planck s quantum theory is able to explain the phenomenon of black body radiation b The wavelength of the first line in Balmer series is 656 nm Calculate the wavelength of the second line in Ralmer series

Physical Chemistry
General66 Which of the following expression for entropy change of an irreversible process dq du d ds T a ds dq T b ds dq T c ds

Physical Chemistry
GeneralColor ealing Brush tool is used to The Patch tool repairs an area with pixels copi The Dodge tool is used to lighten dark areas The Clone stamp tool paints one part of an image over another part of another part of any open document that has the same colour mode The Blur tool is used to selectively blur areas of an image 4 Choose the correct answer 1 The a Clone stamp c Pattern stamp Exercise image o tool is used to remove old marks from an image b Healing brush d All of these

Physical Chemistry
GeneralThe emf of the cell TI TI 0 0001 M Cu2 0 01M Cu is 0 83 V The emf of this cell will be increased by 1 Increasing the concentration of Cu ions 2 Decreasing the concentration of TI 3 Increasing the concentration of both 4 1 2 both

Physical Chemistry
GeneralAn element A crystallises in fcc structure 200 g of this element has 4 12 x 1024 atoms The density N 144 of A is 7 2 g cm3 Calculate the edge length of the unit cell Mas of each atom 1 26 97 x 10 24 cm 3 5 x 10 12 cm 1 2 2 299 9 pm 12x10 4 2 99 cm 48 54X

Physical Chemistry
General4 Conc H SO is 98 by mass and has density of 1 84 g cm 3 What volume of the conc acid is required to make 5 00 litre of 0 5 M H SO4 solution 1 250 cm 2 136 cm 3 13 6 cm 4 136 L

Physical Chemistry
General52 Compounds A B C D E and F are CH CH CHO CH C C CH3 2 CH3CH CH CH3 CH3C CH3 C CH C CH CH3 CH CH and CH C C CH respectively 3 Which of the above compounds upon ozonolysis can give aldehydes A A B D and F B A C and E C B D and E D A only

Physical Chemistry
GeneralCalcium carbonate reacts with aqueous HCl to give CaCl and CO according to the reaction given below CaCO3 s 2HCl aq CaCl2 aq CO3 g H O 1 What mass of CaCl2 will be formed when 250 mL of 0 76 M HCl reacts with 1000 g of CaCO3 Name the limiting reagent Calculate the number of moles of CaCl formed in the reaction

Physical Chemistry
General2 2 4 4g of CO and 2 24 litre of H at STP are mixed in a container The total number of molecules present in the container will be a 6022x1023 c 2 mole b 1 2044x1023 d 6 023x10 4

Physical Chemistry
GeneralD The pressure in a vessel that contained pure oxygen dropped from 2000 torr to 1500 torr in 55 minute as the oxygen leaked through a small hole into a vacuum When the same vessel is filled with another gas the pressure dropped from 2000 torr to 1500 torr in 85 minute What is the molecular weight of gas

Physical Chemistry
GeneralIf C and C denote the specific heats of nitrogen per unit mass at constant pressure and constant volume respectively then JAYC C R 28 B Cp C R 14 C C C R D Cp C 28 R refrigetator If the work done

Physical Chemistry
GeneralN 6 022 x1023 hydroxide OH sulfate SO4 Calculate the mass in grams of 3 25 x1023 molecules of trinitrogen tetraoxide

Physical Chemistry
General2 Calculate the isothermal 1 av ideal gas a V ap gas at T 273K compressibility a for a at P 1 bar and cub T n expansion coefficient 3 P n 1 JP for an ide

Physical Chemistry
GeneralWhich of the following aqueous solution will 72 result in maximum number of moles of metal deposited on passing same charge 1 KI 2 ZnSO4 3 AuCl3 4 CaCl

Physical Chemistry
GeneralQ39 How many grams of methyl alcohol should be added to 10 litre tank of water to prevent it from freezing at 298K K for water is 1 86 K Kg mol A 880 07g B 899 04g C 886 02g D 868 06 g

Physical Chemistry
GeneralWhen ethene reacts with bromine in aqueous sodium chloride Solution The product s obtained is are 1 Ethylene dibromide only 2 Ethylene dibromide and 1 bromo 2 chloro ethane 3 1 bromo 2 chloroethane only 4 Ethylene dichloride only CH CH CA

Physical Chemistry
GeneralWhich of the following statement is true a 6 electrons are present in Mg for which m 0 b 6 electron are present in one p orbital of Mg c Maximum 18 electrons present in M shell 12 d 3 electron present in phosphorous for which 0 m 0 s A a b c d B a be C a c d D c

Physical Chemistry
Generalc NH3 d SF6 149 Under which of the following sets of conditions is a real gas expected to deviate from ideal behaviour I High pressure small volume II High temperature low pressure III Low temperature high pressure b only II PV nRT if pre vu so then expresia c only III const ideal gas d I and III both chaviour a plot between D D was

Physical Chemistry
General3 1 53 x 10 mol A transition metal M forms a volatile chloride which chlorine the formula of the metal chloride will be 1 MC1 2 MC4 4 4 46 x 10 mol has a vapour density of 94 8 If it contains 74 75 AIEEE ONLINE 2015 4 MC13 3 MC15

Physical Chemistry
GeneralH Cl reaction heat of formation of HCI in kJ is a 194 kJ b 97 kJ c 97 kJ d 194 k 2 HCI AH 194 kJ In thi

Physical Chemistry
GeneralPaper chromatography is an example of 1 Partition chromatography Thin layer chromatography 2 3 Column chromatography tion chromatography

Physical Chemistry
Generala 1800 23 2 If mass of one atom is 3 32 x 10g then calculate number of nucleons neutrons and protons present in 2 atoms of the element a 40 b 20 c 10 rent in 9 5 g of PO d 40 NA

Physical Chemistry
GeneralWeight of Agrobtained passed for TO0 second 1 0 11 g 3 10 g when 1 ampere current is in Ag ion solution 2 1 08 g 4 0 05 g
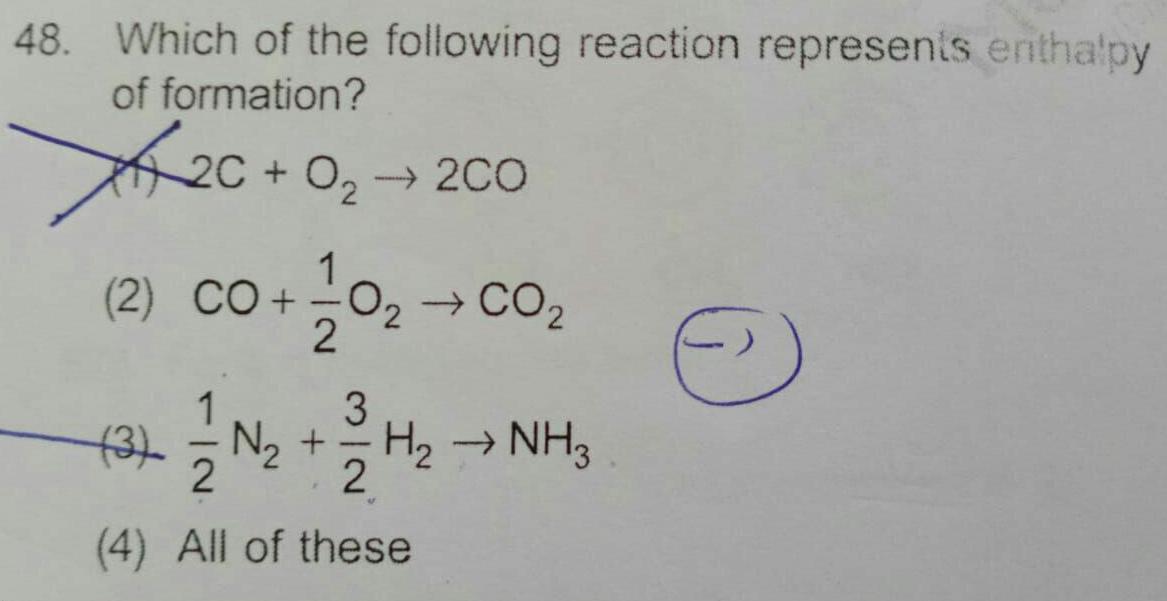
Physical Chemistry
General48 Which of the following reaction represents enthalpy of formation 0 2C 0 200 1 2 CO 0 CO 2 1 3 3 N H NH3 2 4 All of these

Physical Chemistry
General2 In the process of rusting of iron the weight of oxygen that reacts with 5 6 g of iron is A 1 2g B 2 4 g C 3 6 g D 4 8 g

Physical Chemistry
GeneralOne litre of N 2 HCl solution was heated in a beaker When volume was reduced to 600 mL 3 25 g of HC was given out The new normality of solution is a 6 85 c 0 1043 b 0 685 d 6 50

Physical Chemistry
General10 ml of a gaseous compound containing N and O is mixed with 30 ml of H to produce H O and 10 of N g Molecular formula of compound if both reactants reacts completely is A NO C N O3 B NO D N O5

Physical Chemistry
General3 Th 234 disintegrates and emits 63 and 7a particles to form a stable product Find the atomic number and mass number of the stable product and also identify the element IIT 2004

Physical Chemistry
GeneralWhat is the weight of sodium bromate and molarit of solution to prepare 85 5 mL of 0 672 N solution when half cell reaction are i BrO3 6H 6e 10e ii 2BrO3 12H Br 3H O Br 6H 0
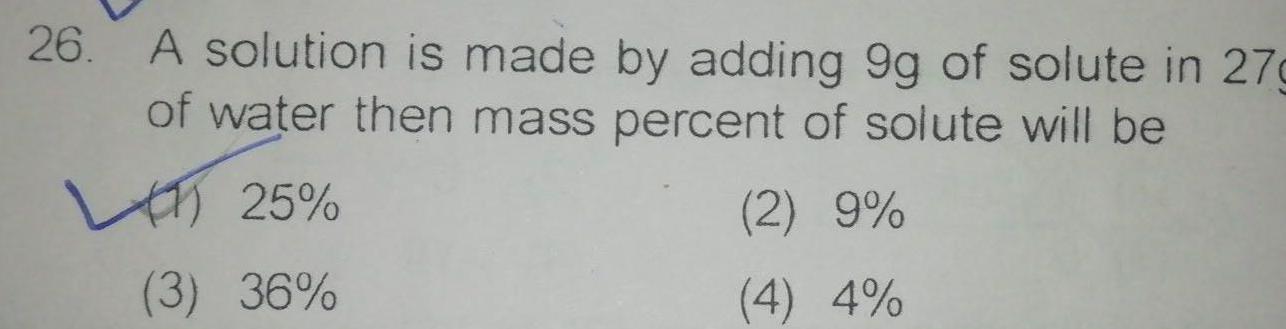
Physical Chemistry
General26 A solution is made by adding 9g of solute in 27g of water then mass percent of solute will be 25 3 36 2 9 4 4

Physical Chemistry
GeneralCalculate the pH of 1 x 10 solution of NH4OH The dissociation constant of NH4OH is 1 85 10 5 mol dm Sol Degree of dissociation a 1 85 10 5 1 10 3 1 36 10 1 Degree of dissociation represents the But V c C concentrations of OH in the solution OH 1 36 10 1 Kb K H O JOH 1 1 85 10 2

Physical Chemistry
GeneralA monoatomic ideal gas undergoes a process in which the ratio of p to Vat any instant is constant and equals to 1 What is he molar heat capacity of the gas 2006 3M 3R 5R 4R 2 b 2 c d 0

Physical Chemistry
GeneralIn presence of fluoride ion Mn2 can be titrated with MnO4 both reactants being converted to a complex of Mn III in presence of F ions A 0 545g of sample containing Mn 304 was dissolved and all manganese was converted to Mn2 The titration in presence of fluoride ion consumed 31 1 mL of KMnO4 that was 0 117 N against oxalate a Write balanced chemical equation for the titration assuming that the complex is MnF4 b What was the of Mn 304 in sample


Physical Chemistry
General40 The data for the reaction A B C is Exp A o B i 0 012 0 035 ii 0 024 0 070 iii 0 024 0 035 iv 0 01200 0 070 The rate law corresponds to the above data is 1 Rate k A B 2 Rate K A B 3 Rate k B 4 Rate k B 4 Initial rate 30 10 0 80 0 10 0 80

Physical Chemistry
GeneralThe correct order of ionic radii of the followin species is XXX 1 Se Br 0 F 2 1 Se 0 Br F X 3 Se Br F 0 X

Physical Chemistry
General74 The wavelength of the radiation emitted when in hydrogen atom electron falls from infinity to stationary state 1 would be Rydberg constant 1 097 x 107 m 1 91 nm 2 9 1 x 10 8 nm 3 406 nm 4 192 nm

Physical Chemistry
GeneralCalculate maximum number of identical A segment X of cellulose obtained on partial hydrolysis has molecular mass 1476 gm On complete acidic hydrolysis mass of the product obtained is 1620 gm Find out the number of glycosidic linkage s present in segment X