General Questions and Answers

Physical Chemistry
GeneralThe value of Planck s constant is 6 63 x 10 34 Js Q 10 The speed of light is 3 x 1017 nm s Which value is closest to the wavelength in nanometer of a quantum of light with frequency of 6 x 10 5 s 1 1 75 3 25 2 10 4 50 miern FR6 63 x 10 34 Js 3 x 1017 nm s 1 1 6 x 1015 s 1 3 de A e gift 1 75 3 25 2 10 4 50

Physical Chemistry
General176 CIVICIFICH Chemistry 18 The extent of ionisation of weak electrolytes 26 At a certa H O 10 this temper 1 10 3 10 67 increases 1 With the increase in concentration of solute 2 On decreasing the temp of solution 3 On addition of excess of water to the solution

Physical Chemistry
GeneralMetal oxides that dissolve in wate r are called Alkalis and Metal oxid es that are soluble in water further dissolve to form Metal Hydroxides Differentiate between Alkalis and Metal Hydroxides regarding their s olubility in water

Physical Chemistry
GeneralHaemoglobin contains 0 33 of iron by weight The molecular weight of haemoglobin is approximately 67200 The number of iron atoms at wt of Fe 56 present in one molecule of haemoglobin are 1 1 2 2 3 4

Physical Chemistry
General15 For the reaction CuSO4 5H O Which one is correct representation 1 K Pho 3 K K RT Ses CuSO4 3H O 2H O 2 K H 012 4 All of these CE0017

Physical Chemistry
GeneralCE0003 In the chemical reaction N 3H 2NH at equilibrium state whether 1 Equal volumes of N H are reacting 2 Equal masses of N H are reacting 3 The reaction has stopped 4 The same amount of ammonia is formed as is decomposed into N and H in the same time CE0004 10

Physical Chemistry
GeneralDissolving 120 g of a compound of mol wt 60 in 1000 g of water gave a solution of density 1 12 g mL T molarity of the solution is 1 1 00 M 2 2 00 M 3 2 50 M car 4 00 s

Physical Chemistry
General3 d 11 In Cu NH3 4 the hybridisation of central atom and number of unpaired electron can be respectively NCERT Pg 254 2 dsp 1 4 dsp 2 MH and TI H O are octahedral e it depends 1 sp 2 3 sp 3

Physical Chemistry
GeneralOn analysis a certain compound was found to contain iodine and oxygen in the ratio of 254 gm of iodine at mass 127 and 80 gm oxygen at mass 16 What is the formula of the compound 1 10 2 1 0 3 LO

Physical Chemistry
General3 On addition of excess of water to the solution 4 On stirring the solution vigorously IE0020 9 If K of HCN 4 x 10 10 then the pH of 2 5 x 10 molar HCN aq is 1 4 2 2 4 7 3 0 47 4 5 0 IE0021

Physical Chemistry
GeneralPleasee tell why in ncert it is written that KMnO4 oxidises oxidation the sulphate SO42 to persulphate S2082 in re is a increase in oxidation number but in this case suphu r exhibit 6 oxidation state in both compound pleasee clear my doubt

Physical Chemistry
General24 Substance A tetramerises in water to the extent of 80 A solution of 2 5 g of A in 100 g of water lowers the freezing point by 0 3 C The molar mass of A is K for water 1 86 K kg mol 1 122 2 31 3 244 4 62

Physical Chemistry
General16 23 If any solute A dimerises in water at 1 atm pressure and the boiling point of this solution is 100 52 C If 2 moles of A is added to 1 kg of water and k for water is 0 52 C molal calculate the percentage association of A 1 50 3 25 2 30 4 100

Physical Chemistry
GeneralAll India Major Test Series Phase I II Major Test 45 39 10 af faa 2 CH 4 NF3 CHEMISTRY Which of the following molecules has the Q 6 maximum dipole moment 1 CO 3 NH3 2 CH4 4 NF3 1 CO 3 NH3 9 2020

Physical Chemistry
General1 Diamond has a three dimensional structure of C atoms formed by covalent bonds The structure of diamond has face centred cubic lattice where 50 of the tetrahedral voids are also occupied by carbon atoms The number of carbon atoms present per unit cell of diamond is Your Answer

Physical Chemistry
Generalons weight of Pre Medical Chemistry 177 Build Up Your Understanding 11 If the molar concentration of MgCl is 1 5 x 10 mol L the concentration of chloride ions in g ion L is

Physical Chemistry
GeneralA gaseous hydrocarbon gives upon combustion 0 72 gm of water and 3 08 gm of CO The empirical formula of the hydrocarbon is 1 C6Hs 2 C Hx 3 C H 4 C H4

Physical Chemistry
General2 P P 2 3 P P 4 Can t say anything 25 The internal energy of a gas in an adiabatic process is 25 a given by U a bPV find y 1 3 a 1 a b 1 a b 1 2 b 1 4 a b 1 1 P P 3 P P 1 3 a 1 a b 1 a y El Y 2 P P feff U a bPV RT 4 The H T T 2 b 1 a b 1

Physical Chemistry
General32 In a 0 2 molar aqueous solution of a weak acid HX the degree of ionization is 0 3 The freezing point IT i Ko the solution will be nearest to 1 0 48 C 3 0 36 C 2 0 48 C 4 0 26 C

Physical Chemistry
GeneralES0058 60 The electric potential V is given as a function of distance x metre by V 5x 10x 9 volts The value of eletriec field at x 1 m is 1 20 V m 2 6 V m 3 11 V m 4 zero ES0059 65

Physical Chemistry
General20 electric field E is constant in both magnitude and direction Consider a path of length d at an angle 60 with respect to field lines as shown in figure The potential difference between points 1 and 2 is E 1 d sin 60 2 Ed cos 60 3 4 Ed cos 60 sin 60 W 10 FS0063 F

Physical Chemistry
General4 300 Rin2 24 From the following V T diagram we can conclude P h T sule 3 300 R In2 24 V T 3 1 P P 2 P P 3 P P 4 Can t say anything 25 The internal energy of a gas in an adiabatic process is 25 1 P P 3 P P 2 600 Rin2 4 300 Rin2 fida T 2 P P 4 d ff U a bPV R

Physical Chemistry
GeneralWhich of the following statements are correct a Helium has the highest first ionisation enthalpy in the periodic table b Chlorine has less negative electron gain enthalpy than fluorine c Mercury and bromine are liquids at room temperature d In any period atomic radius of alkali metal is

Physical Chemistry
GeneralA mixture of two liquids A and B having boiling point of A is 70 C and boiling point of B is 100 C distills at 101 2 C as single liquid hence this mixture is 1 Ideal solution HO 2 Non ideal solution showing ve deviation 3 Non ideal solution showing ve deviation 4 Immiscible solution

Physical Chemistry
General1 0 g of magnesium is burnt with 0 56 g O in Q 5 a closed vessel Which reactant is left in excess and how much At wt Mg 24 O 16 1 Mg 0 16 g 3 Mg 0 44 g 2 0 0 16 g 4 0 0 28 g 1 0 g 0 56 g 0 dall 24 g Mg 1 Mg 0 16 g 3 Mg 0 44 g SPACE FOR ROUGH WORK 0 16 2 0 0 16 g 4 0 0 28 g

Physical Chemistry
GeneralA P 235y 125 xy mm of Hg P is partial pressure of A x is mole fraction of B in liquid phase in the mixture of two liquids A and B and y is the mole fraction of A in vapour phase then Pg in mm of Hg is 2 O 4 125 1 235 3 110

Physical Chemistry
General27 Equimolar solution of non electrolyte in the same solvent have 1 Same boiling point and same freezing point 2 Different boiling point and different freezing point 3 Same boiling point but different freezing point 4 Same freezing point but different boiling point

Physical Chemistry
General2 Two concentric spheres of radi R and r have similar charges with equal surface charge densities G What is the electric potential at their common centre 1 0 0 2 R 1 50 3 R 1

Physical Chemistry
Generalof helium gas are taken over the cycle ABCDA as shown in the V T diagram V m A 2 I D B T K 300K 500K 22 Assuming the gas to be ideal the work done on the gas in 2 taking it from A to B is 1 400 R 2 500 R 3 200 R 4 Zero 3 The work done on the gas in taking it from D to A is 1 600 R In2 2 600 Rin2 3 300 R In2 4 300 Rin

Physical Chemistry
GeneralEXERCISE I Conceptual Questions INTRODUCTION 1 The formula weight of H SO is 98 The weight of the acid in 400mL of 0 1 M solution is 2 3 92 g 1 2 45 g ni 11

Physical Chemistry
GeneralWhich statement is true A ring of radius R carries a uniformly distributed charge Q A point charge q is placed on the axis of the ring at a distance 2R from its centre and released The particle executes simple harmonic motion along the axis of the ring ii Electrons move from a region of higher potential to that of lower potential 1 only i 2 only ii 3 i ii 4 none of them ES0065 70 P

Physical Chemistry
General1 The electric potential and electric field at a poi due to a point charge are 600 V and 200 N respectively Then magnitude of the point charg should be 1 3 C 2 30 C 3 0 2 C 4 0 5 ES006

Physical Chemistry
GeneralGR0161 9 Assuming that the gravitational potential energy of an object at infinity is zero the change in potential energy final initial of an object of mass m when taken to a height h from the surface of earth of radius R is given by 1 GMm R h 3 mgh 2 4 GMmh R R h GMm R h GR0162

Physical Chemistry
Generalcharge As a result the force of attraction of the nucleus for the electrons decreases and hence the ionic radii increase 3 14 What is the significance of the terms isolated gaseous atom and ground state while defining the ionization enthalpy and electron gain enthalpy fillonization ontholny is the mini amount of normi required to remove the most loosely bound

Physical Chemistry
General9 Escape velocity for a projectile at earth s surface is V A body is projected form earth s surface with velocity 2 V The velocity of the body when it is at infinite distance from the centre of the earth is 1 V 2 2V 3 2 V 4 3 V GROO89

Physical Chemistry
GeneralALLEN 94 A satellite is orbiting earth at a distance r Variations of its kinetic energy potential energy and total energy is shown in the figure Of the three curves shown in figure identify the type of mechanical energy they represent Energy 0 2 3 1 1 Potential 2 Kinetic 3 Total 2 1 Total 2 Kinetic 3 Potential 3 1 Kinetic 2 Total 3 Potential

Physical Chemistry
GeneralA crystalline solid AB has NaCl type structure with radius of Bion is 250 pm Which of the following cation can be made to slip into tetrahedral site of crystals of A B 1 P radius 180 pm 2 Q radius 56 pm 3 R radius 200 pm 4 St radius 150 pm

Physical Chemistry
GeneralIf NA is Avogadro number then the number of valence electrons in 4 2 g of N ions is 1 2 4 NA 2 4 2 NA 3 1 6 NA 4 3 2 NA

Physical Chemistry
General2 The earth revolves around the sun in one year If distance between them becomes double the new time period of revolution will be 1 4 2 years 3 4 years 2 2 2 years 4 8 years MO2 EXERCISE PAS

Physical Chemistry
Generalons otal ves ical 95 Pre Medical Physics 213 The mean distance of mars from sun is 1 5 times that of earth from sun What is approximately the number of years required by mars to make one revolution about sun 1 2 35 years 2 1 85 years

Physical Chemistry
GeneralIn normal spinel structure there is a closed packed array of O2 ions The trivalent cations are present in 1 75 of octahedral voids 2 50 of octahedral voids 3 12 5 of tetrahedral voids 4 25 of octahedral yoids

Physical Chemistry
GeneralAns Statement d is incorrect The correct statement is Removal of electron from value is difficult than from orbital having higher n value All other statements are correct Q 3 38 Considering the elements B Al Mg and K the correct order of their metallic character is a B Al Mg K b Al Mg B K c Mg Al K B d K Mg Al R Ans In a period electronegativity increases and hence the metallic character decreases as we move from

Physical Chemistry
GeneralAmon non conducting ring is of radius 0 5 m 1 11 x 10 10 coulombs charge is non uniformly distributed over the circumference of ring which produces electric field E around itself If 0 is the centre of the ring then the value of 1 0 Ede is 1 2 V 2 2 V 3 1 V 4 zero

Physical Chemistry
GeneralA crystalline solid AB adopts sodium chloride type structure with edge length of the unit cell as 745 pm and formula mass of 74 5 a m u The density of the crystalline compound is 1 2 16 g cm 3 188 g cm 2 0 99 g cm 4 1 197 g cm

Physical Chemistry
GeneralES0036 given by hrough a plane is ES0041 43 The electric field in a region of space is given by E 51 21 N C The electric flux through an area of 2 m lying in the YZ plane in S I units is 1 10 2 20 3 10 2 4 2 29 FS001
Physical Chemistry
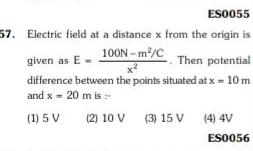
GeneralES0055 57 Electric field at a distance x from the origin is 100N m C given as E Then potential difference between the points situated at x 10 m and x 20 m is 1 5 V 2 10 V 3 15 V 4 4V ES0056

Physical Chemistry
GeneralES004 48 What is the electric potential at a distance from the centre inside a conducting sphere having charge Q and radius R 1 4 R 49 19 46 x 4 zero ES004 Certain positive charge is given to a conductor The

Physical Chemistry
GeneralNEET UG 2019 Odisha rth 28 The time period of a geostationary satellite is 24 h at a height 6RE RE is radius of earth from surface tre of earth The time period of another satellite whose height is 2 5 RE from surface will be 1 6 2 h 2 12 2 h 59 ace the 24 3 h 12 4 h

Physical Chemistry
General3 me only GR0086 87 Near the earth s surface time period of a satellite is 1 4 hrs Find its time period if it is at the distance 4R from the centre of earth 1 32 hrs 2 2 hrs 4 me and r 3 8 2 hrs 4 16 hrs GRO087

Physical Chemistry
General1 162m x 10 Nm C 2 162m x 10 Nm C 3 162m x 10 6 Nm C 4 162m x 106 Nm C ES0043 5 A point charge is placed at a distance perpendicular to the plane and above the centre of a square of side a The electric flux through the square is 1 0 9 3 4 0 2 EO 9 4 60