General Questions and Answers

Physical Chemistry
General67 When heating PCI then it decompose PCL and Cl in form of gas The vapour density of gas mixture is 70 2 and 57 9 at 200 C and 250 C The degree of dissociation of PC at 200 C and 250 C is 1 48 50 80 2 60 70 3 70 80 4 80 90 C50076

Physical Chemistry
General2 17 Among the following the correct statement is Q 17 1 Between NH and PH NH3 is better electron donor because the lone pair of electrons occupies spherical s orbital and is less directional 2 Between NH and PH PH is better electron donor because the lone pair of electrons occupies sp orbital and is more directional 3 Between NH and PH NH3 is better electron donor because the lone pair of electrons occupies sp orbital and is more directional 0 4 Between NH and PH PH is better electron donor because the lone pair of electrons occupies spherical s Dital and is less directional A 1 NH TT PHy V NH T i Stel Ed a s 2 NH HTT PHI PHIN 3D G za sva p the nitute 3 NH HTT PHI 7 Nha v au Bea aran ifas var p ger afire fragt 4 NH FR PHI R PH T i SEO B N af afta id t

Physical Chemistry
Generalen in brackets 10 10 ce to the passage 4 Q EC0007 salt of 0 01 M 11 EC0013 The specific conductance of a 0 01 M solution of KCI is 0 0014 ohm cm lat 25 C Its equivalent conductance cm ohm eq is 1 140 3 1 4 2 14 4 0 14 EC0014 MODULE44ELECTROCHEMISTRY 22 EXP

Physical Chemistry
Generaland O at duced so that perature and Le Chatelier CALCULATION OF DEGREE OF DISSOCIATION BY V D METHOD 66 Vapour density of PCI is 104 25 at t C Then degree of dissociation of PCI is Mw 208 5 1 20 2 0 3 30 4 15

Physical Chemistry
Generala Temperature b Nature of solute c Pressure d Nature of solvent 14 200 ml water is added to 500 ml of 0 2 M solution What is the molarity of the diluted solution a 0 5010 M b 0 2897 M c 0 7093 M d 0 1428 M

Physical Chemistry
GeneralA gaseous mixture containing He CH4 and SO was allowed to effuse through a fine hole then find what molar ratio of gases coming out initially Given mixture contains He CH and SO in 1 2 3 mole ratio 1 2 2 3 3 11 3 2 2 2 3 4 4 4 3 Q 4 He CH4 i SO a v tu fas TH ROG i THETH HARIN BA I THI H CH 31 SO ff 1 2 3 1 1 2 2 3 3 11 3 2 2 2 3 4 4 4 3

Physical Chemistry
General53 Copper of fixed volume V is drawn into wire Q 153 3 ta of length When this wire is subjected to a constant force F the extension produced in the wire is AC Which of the following graphs is a straight line 1 AC versus 1 2 A versus 3 A versus 1 2 4 A versus AREN mier Initi A 1 A versus 1 2 A versus 3 A versus 1 4 A versus 3 F A a

Physical Chemistry
GeneralCalculate the standard electrode potential E for e Fe From the given standard electrode potentials value of two half cells Fe 2e Fe 3e Fe E 0 036 V A B 2e Fe E 0 440 V C 0 772 V 0 404 V 0 404 V

Physical Chemistry
General157 When a stretched wire and a tuning fork are sounded together 5 beats per seconds are Q 157 produced when length of wire is 95 cm or 100 cm frequency of the fork is 1 90 Hz 3 105 Hz 2 100 Hz 4 195 Hz ier 19 3 411 Hz 4 448 Hz g are an af fy 5 95 cm T100 cm for at argfa eft 1 90 Hz 2 100 Hz 3 105 Hz 4 195 Hz

Physical Chemistry
General3 PQ3 2 7 x 10 The correct order of decreasing molar solublity is 76 1 1 2 3 2 2 1 3 3 3 2 1 4 2 3 1 IE0081 0 The K value for Gd OH is 2 8 x 1023 Find the pH of saturated solution of Gd OH 1 6 08 2 5 08 3 8 47 4 4 08

Physical Chemistry
Generaled anoth question ue itutor In the video lecture it was a bit lengthy Pleas e verify if my method and assumption for the two unknow In compounds is correct a to oxygen G Hydrogen and are known to farm two compound The hydrogen content in one of these is 5 93 while in the other it is 11 2 show that this data illustrates the law of multiple proportions solution We know the two compound made by the given elements is hydragen and oxygen are H O and H O weight of H 29 29 H O H O weight of oxygen 16g 32g Ratio for oxygen 16 32 12 Hence Proved

Physical Chemistry
GeneralALLEN 53 AB dissociates as 2AB g 2A g B g When the initial pressure of AB is 500 mm t total pressure becomes 625 mm when th equilibrium is attained Calculate K for the reacti assuming volume remains constant

Physical Chemistry
General1 CHEMISTRY The most probable radius in pm for finding Q 1 the electron in He is 1 0 0 3 26 5 2 52 9 4 105 8 Hea fafar pm 1 0 0 3 26 5 2 52 9 4 105 8

Physical Chemistry
General32 If some He gas is introduced into the equilibrium PCI PCI Cl at constant pressure and temperature then equilibrium constant of reaction 1 Increases 2 Decreases 3 Unchanged 4 Nothing can be said CF0036 37 In the reac 2 mol ea 1 L flask A 1 3 Q F P Q

Physical Chemistry
Generalgalvanometer is 5 div mA and its voltage sensitivity angular deflection per unit voltage applied is 20 div V The resistance of the galvanometer is 1 40 2 3 250 2 2 250 4 500 2 5 div mA afte teen een de 1 402 3 250 02 ofta fata 20 div V 1 fer 2 250 4 500 2

Physical Chemistry
GeneralMood is the attitude attitude atmosphere character symbolism 5 6 7 a text creates 8 19 10 son s F

Physical Chemistry
GeneralConsider the following cell reaction 2Fe s O g 4H aq 2Fe2 aq 2H O D E 1 67 V At Fe 10 3 M P O 0 1 atm and pH 3 the cell potential at 25 C is 1 47 V 1 77 V 1 87 V

Physical Chemistry
GeneralThe oxidation of succinate to fumarate in the TCA Cycle has a standard state free energy change Go of 5 8 kJ mole What is the change in standard reduction potential o for this reaction Round the answer to two decimal places

Physical Chemistry
GeneralWhich one is a wrong statement for the given manometer P Patm U 0000000000 Manometer fluid Select one a Measures pressure difference between two points in the line b Density of the manometer fluid should be known O c P is greater than P Od This is an example of an open end manometer

Physical Chemistry
General4 None of these CE007 PHYSICAL EQUILIBRIUM 64 For the equilibrium reaction H 0 0 H 0 What happens if pressure is applied 1 More water evaporates 2 The boiling point of water is increased 3 No effect on boiling point 4 None of the above CE007

Physical Chemistry
General0 pH of water is 7 When any substance Y is dissolved in water then pH becomes 13 Substance Y is a salt of 1 Strong acid and strong base 2 Weak acid and weak base 3 Strong acid and weak base 4 Weak acid and strong base 8

Physical Chemistry
General1 If the maximum concentration of PbCl in water is 0 01 M at 298 K its maximum concentration in 0 1 M NaCl will be 1 4 x 10 M 3 4 x 10 M 2 0 4 x 104 M 4 4 10 M 150076

Physical Chemistry
GeneralA student heats solid copper sulphate in a dry test tube and reports the following observations The observati which is correctly noticed is A it breaks with cracking sound B a reddish brown gas is evolved C water droplets collect in the cooler parts of the test tube D a dark blue residue is formed

Physical Chemistry
Generalession 7 In water 1 Na PO 3 NaNO IE0047 47 A salt X is dissolved in water of pH 7 The resulting solution becomes alkaline in nature The salt is made IE0048 2 CH COONa 4 Both 1 and 2 IE0053 up of 1 A strong acid and strong base 2 A strong acid and weak base 3 A weak acid and weak base 4 A weak acid and strong base IF0054

Physical Chemistry
General1 gram of a carbonate M CO3 on treatment with excess HCl produces 0 01186 mole of CO The molar mass of M CO in g mol is 1 118 6 2 11 86 3 1186 4 84 3 CM 100 SEJOUD

Physical Chemistry
GeneralThe zinc content of a 1 62 g ore sample was determined by dissolving the ore in HCI which reacts with the zinc The excess HCI is then neutralized with with NaOH The reaction of HCI with Zn is shown Zn s 2HCl aq ZnCl aq H g The ore was dissolved in 150 ml of 0 600 M HCI and the resulting solution was diluted to a total volume of 300 mL A 20 0 ml aliquot of the final solution required 9 95 mL of 0 546 M NaOH to neutralize the excess HCI What is the mass percentage w w of Zn in the ore sample

Physical Chemistry
General4 None of these 0 2 mole of HCI and 0 1 mole of barium chloride were dissolved in water to produce a 500 mL solution T molarity of the Cl ions is 1 0 06 M 2 0 09 M 3 0 12 M 4 0 80 M

Physical Chemistry
Generalhe partial 49 Evaluate K for the reaction H 1 2H1 If 0 2 and 2 moles each of H and I are taken initially At equilibrium moles of Hi are 2 1 2 5 2 4 3 0 25 If partial to twice sin atm at 4 1 0 CE0055

Physical Chemistry
GeneralThe mass of oxygen that would be required to produce enough CO which completely reduces 1 6 kg Fe at mass Fe 56 is Fe O3 3C0 2Fe 3CO2 1 240 gm 3 720 gm 2 480 gm 4 960 gm

Physical Chemistry
GeneralInitially 22 2 g of calcium chloride are d issolved in water to make a 500 mL sol ution Then the solution is again diluted upto 2000 mL Calculate the molarity of ct ions in the final solution if the 80 of the given salt dissociates in the final so lution

Physical Chemistry
General41 A boy of mass 50 kg jumps to a height of 0 8 Q 141 m from the ground then momentum transferred by the ground to boy is 1 400 kg m s 2 200 kg m s 3 800 kg m s 4 500 kg m s 50 kg 0 8 m das cena o verrata 1 400 kg m s 2 200 kg m s Medici 3 800 kg m s 4 500 kg m s

Physical Chemistry
General5 A satellite S is moving in an elliptical orbit Q 145 around the earth The mass of the satellite is very small compared to the mass of the earth Then 1 the acceleration of S is always directed towards the centre of the earth 2 the angular momentum of S about the centre of the earth changes in direction but its magnitude remains constant 3 the total mechanical energy of S varies periodically with time 4 the linear momentum of S remains constant in magnitude itut 4 eat a cha 1 2 gefta femfeta vem f gea 1 Ser afea gen 1

Physical Chemistry
GeneralHow many grams of NH can be produced from 5 0 g nitrogen and 3 0 g hydrogen im the following reaction Atomic weight of N 14 0 and H 1 0 2 0 g 6 1 g 178 no correct answer given N g 3 H g 2 NH g

Physical Chemistry
GeneralUSE CODE BAHUBALI FOR 10 OFF ON PLUS ICONIC SUBSCRIPTION 1 84 gram mixture of CaCO and MgCO on heating gives CO Volume of CO obtained is measured to be 448 mL at STP mass of CaCO in mixture is 1 0 5 gram 2 0 84 gram 3 0 92 gram 4 1 00 gram

Physical Chemistry
General24 lonic product of water is equal to 1 Dissociation constant of water x H O 2 Dissociation constant of water x H 3 Product of HO and H 4 Product of OH and H 15000
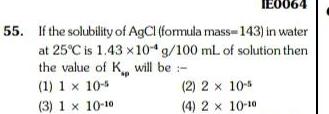
Physical Chemistry
GeneralIE0064 55 If the solubility of AgCl formula mass 143 in water at 25 C is 1 43 x 104 g 100 mL of solution then the value of K will be 1 1 x 10 2 2x 10 5 3 1 x 10 10 4 2 x 10 10

Physical Chemistry
GeneralEET PHYSICS 140 The rear side of a truck is open and a box of Q 140 mass 20 kg is placed on the truck 4 m away from the open end If u is 0 15 and g is 10 m s 2 and the truck starts from rest with an acceleration of 2 m s 2 on a straight road then the box will fall off the truck when it is at a distance of x metre from the starting point The value of x is All India Major Test Series Phase l II Major Test 45 39 10 09 2020 20 kg 1 4 m 3 16 m 2 8 m 4 32 m 4 m 1 4 m 3 16 m 0 15 10 ms2en 2 ms Fra 1 fandt fag 2 8 m 4 32 m xam

Physical Chemistry
Generalsp d 3 Select incorrect match for M H O complex Metal ions 1 Mn 2 V 3 Ni 4 Ti Electronic configuraiotn teg CFSE 040 1 24 1 6A 0 840 3 4 F M C

Physical Chemistry
General23 For a reaction N 3H 2NH the value Ke does not depends upon a Initial concentration of the reactants b Pressure c Temperature d Catalyst 1 Only c 3 a b d 2 a b c 4 a b c d CE002

Physical Chemistry
GeneralMalonic acid is heated to form a product which is treated with NOOH form sodium salt This solt is electrolysed in aqueous medium to form product A If malonic acid is directly treated with NGOH and electrolysed in aqueous medium it forms B The difference in molar mass of A and B is Only one correct answer A 4 C 2 12

Physical Chemistry
GeneralPhosphine PH decomposes to produce P g and H g What would be the change in volume when ml of PH g is completely decomposed 1 50 ml 2 500 ml 3 75 ml

Physical Chemistry
Generalat 2000 K is 8 and 10 respectively calculate the emissivity of IBB Ideal black body 1 0 2 2 0 4 4 0 8 3 0 6 8 10 se afore 1 0 2 3 0 6 2 0 4 4 0 8

Physical Chemistry
GeneralSelect all that apply which of the following aqueou solutions is isotonic with biologist fluids A 4 5 g NaCl in 500 ml total volume B 20 g dextrose in 200 ml Total volume C 0 9 g nacl in 50 ml total Volume D 10 g dextrose in 200 ml total volume

Physical Chemistry
GeneralVapour density of a compound with respect to ethane is 4 07 The compound has 68 55 carbon 4 92 hydrogen and rest oxygen What is the molecular formula of the compound 1 C H O 3 C H1002 2 C H O 4 C H O

Physical Chemistry
General24 For any reversible reaction if concentration of reactants increases then value of equilibrium constant 1 Depends on amount of concentration 2 Unchanged 3 Decreases 4 Increases 29 2 20

Physical Chemistry
GeneralStructure of a mixed oxide is cubic close packed c c p The cubic unit cell of mixed oxide is composed of oxide ions One fourth of the tetrahedral voids are occupied by divalent metal A and the octahedral voids are occupied by a monovalent metal B The formula of the oxide is 1 A B304 3 ABO 2 AB O 4 A BO mier 149 Holod 0 0 1 A B304 3 ABO 34 Ra ART HIS T Red B 2 AB O 4 A BO

Physical Chemistry
General4 Oxidizing in a and b Among the following complexes the one which shows Zero crystal field stabilization energy CFSE is 1 Mn H O 3 Co H O 2 2 Fe H O 4 Co H O Q 8 4 a b A CFS 1 Mn H O 3 Co H O F R 2 Fe H O 4 Co H O 1

Physical Chemistry
Generala H O O H O 20 b H O Ag O 2Ag H O O Role of hydrogen peroxide in the above reactions is respectively 1 Oxidizing in a and reducing in b 2 Reducing in a and oxidizing in b 3 Reducing in a and b 4 Oxidizing in a and b Q 7 a H O O H O 20 b H O Ag 0 2Ag H O O 1 a 2 a 3 a 4 a b b b b BRIGGS cal

Physical Chemistry
GeneralIn an organic compound of molar mass 108 gm mol C H and N atoms are present in 9 1 3 5 by weight Molecular formula can be 1 C H N 2 C H ON 3 C H N 4 C H N

Physical Chemistry
GeneralHow many moles of magnesium phosphate Mg PO will contain 0 25 mole of oxygen atoms 1 3 125 102 2 1 25 102 3 2 5 102 4 0 02 USE CODE BAHUBALI FOR 10 OFF ON PLUS ICONIC SUBSCRIPTION