Solid state Questions and Answers

Physical Chemistry
Solid stateScandium oxide Sc O3 crystallises with the oxide ions in a closed packed array with the scandium ions in octahedral holes What fraction of the octahedral holes are filled 1 All 2 2 3 1

Physical Chemistry
Solid stateThe density and edge length values for a crystalline element with fcc lattice are 10 g cm and 400 pm respectively The number of unit cells in 32 g of this crystal is A 8 1023 B 5 x 1022 C 8 1022 D 5 tune of defect observed in stoichiometric compounds is Schottky defect Schottky defect occur mainly in ionic

Physical Chemistry
Solid stateIn a spinel structure AB204 the divalent cations A occupy x of the tetrahedral voids while trivalent cations B occupy y of the octahedral voids and z of the tetrahedral voids Then the value of y z x is Answer 00 01 02 03 04 05 06 07 8 09

Physical Chemistry
Solid stateQuestion 25 Which is are correct statement about zinc blende structure Options a The number of first neighbors of S is 4 b The maximum distance between Zn is a3 where a edge length of unit cell a 3 c If all tetrahedral voids occupied by Zn then C N of S is 8 d If all tetrahedral voids occupied by Zn then C N change from 4 4 to 8 8

Physical Chemistry
Solid stateThe radius of a divalent cation A2 is 94 pm and of divalent anion B2 is 146 pm Th compound AB has a Rock salt structure c Antifluorite structure b Zinc blende structure d Caesium chloride like structure

Physical Chemistry
Solid stateIn a structure oxide ions are cubical closest packed whereas t th of tetrahedral voids are 1 8 1 occupied by A cation and of octahedral voids 2 are occupied by B 3 cations The formula for this compound is 1 ABO 4 2 AB 04 4 ABO

Physical Chemistry
Solid stateElement x y and z crystallize in primitive face centered and body centered unit cell respectively What would be the correct order of metallic radii if volume of each unit cell is same 1 y 3 r r r 2 ry r r 4

Physical Chemistry
Solid state6 In cubic lattice atoms are arranged A at corners B at face centers C at edge and body centre and 2D at triads tetrahedral voids then the body diagonal touches 1 2A 2C 2D 3 2A B 2C 2 2A 2B C 4 2A C 2D in fc

Physical Chemistry
Solid stateequations is most applicable 1 AH AE 2 AH AS 3 AH AE The void space in a primitive unit cell is 1 48 void space 2 24 void space 3 96 void space In chelate therapy lead toxicity is removed by using the ligand Coo 4 Total W 0 4 50 void space

Physical Chemistry
Solid stateSquare packed sheets are arranged one on the top of the other such that a sphere in next layers rests on the top of spheres in previous layer Identify the type of arrangement and find the coordination number 1 simple cubic 8 2 simple cubic 6 3 body centerede cubic 8 4 face centered cubic 12

Physical Chemistry
Solid stateConsider an ionic solid MX with NaCl structure Construct a new structure Z whose unit cell is constructed from the unit cell of MX following the sequential instructions given below Neglect the charge balance i Remove all the anions X except the central one ii Replace all the face centered cations M by anions X iii Remove all the corner cations M iv Replace the central anion X with cation M The value of number of anions number of cations in Z is

Physical Chemistry
Solid state09 Indium Antimonide InSb is a semiconductor material and it has the zinc blende structure The atomic radii of In and Sb atoms are in 1 44 and rsb 1 36 respectively Assume that the ions as touching hard spheres Molar masses In 114 8 g and Sb 121 7 g a Draw a sketch of a InSb unit cell b How many In2 ions belong to a unit cell c What is the coordination number of In ions d How many Sb ions belongs to a unit cell e Calculate the unit cell constant of the InSb unit cell www f Calculate the density of InSb

Physical Chemistry
Solid stateExample 1 9 In a CPS close packed structure of mixed oxides it is found that lattice has 02 oxide ions and one half of octahedral voids are occupied by trivalent cations A and one eighth of tetrahedral voids are occupied by divalent cations B2 Derive formula of the mixed oxide 3

Physical Chemistry
Solid state29 A crystal is made of particle x y and z x forms f c c packing y occupies all the octahedral voides of x and z occupies all the tetrahedral voides of x If all particle along one body diagonal are removed the formula of the crystal would be 1 XYZ 2 3 X8Y4Z5 2 X YZ 4 X5Y4Z8
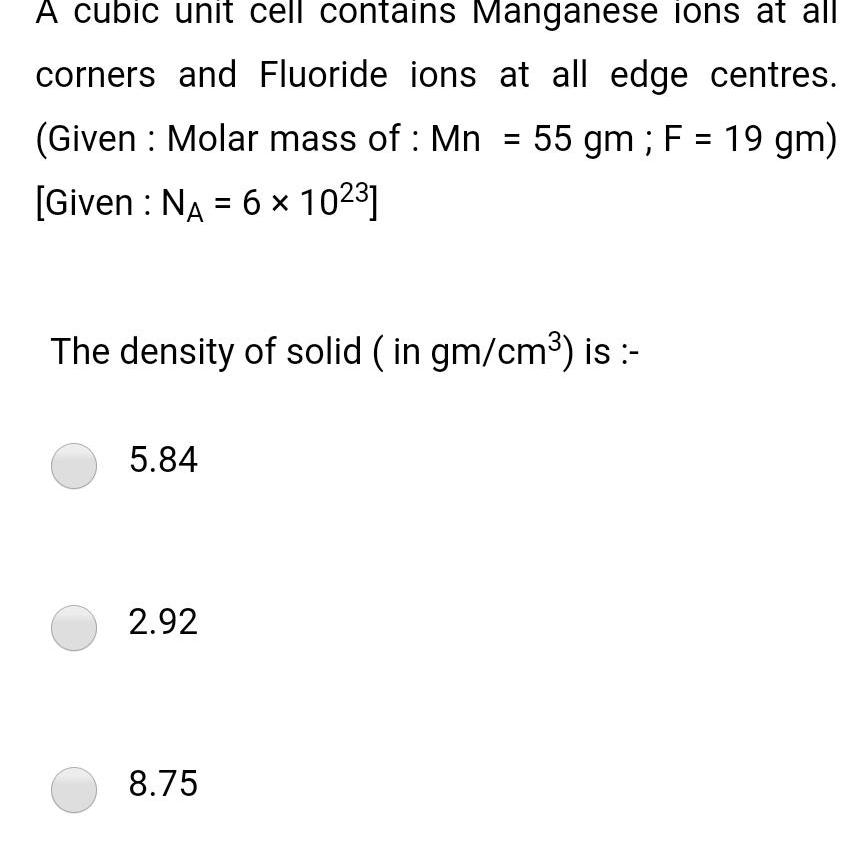
Physical Chemistry
Solid stateA cubic unit cell contains Manganese ions at all corners and Fluoride ions at all edge centres Given Molar mass of Mn 55 gm F 19 gm Given NA 6 1023 The density of solid in gm cm is 5 84 2 92 8 75

Physical Chemistry
Solid statePotassium crystallizes in BCC with unit cell edge length of 500 pm Identify correct statement s about this solid Given N 6 10 Shortest distance between two potassium atoms is 4 33 The number of next nearest neighbours of each potassium atoms are 8 Shortest distance between two potassium atoms is 3 54 The density of this solid is 10 4 gm cm K 39

Physical Chemistry
Solid stateA metal A exists in the bcc structure having uncovered length between the atoms along the edge in an unit cell equal to 1 2 If molar mass of the metal is 307 2 gm mole then calculate density of the crystal in gm ml Use NA 6 x 1023 and 3 1 7

Physical Chemistry
Solid stateAB crystal has CsCl type structure If edge length of unit cell is 100 pm then nearest distance between cation and anion is mf w x 1 50 pm 3 1732 pm 2 100 pm 4 86 6 pm

Physical Chemistry
Solid stateIn ionic crystal AB if radius of cation and anion is 30 pm and 60 pm respectively then crystal structure is 1 ZnS type 3 CSCL type 2 NaCl type glt 4 B O type 12

Physical Chemistry
Solid stateCalcium metal crystallises in a face centered cubic lattice with edge length of 0 556 nm Calculate the density of the metal State Atomic mass of calcium 40 g mol N 6 022 x 1023 atoms mol 2 A

Physical Chemistry
Solid stated bcc and hcp 3 The number of octahedral void in bcc structure is a 0 b 1 c 2

Physical Chemistry
Solid stateestiloss to songor ai er Silver Atomic weight 108 g mol has a density of 10 5 g cm The number of silver atoms on a surface of area 10 2 m can be expressed in scientific notation as y 10 The value of x is de Mil Vot A 2 B 3 D 7 adh gnieu beau C 5 Made atan 106 10 to 6 29 definito

Physical Chemistry
Solid state70 How many nearest and next nearest neighbours respectively does potassium have in bcc lattice a 8 8 educ c 6 8 T 71 b 8 6 ng of d 6 6

Physical Chemistry
Solid stateIn a H like atom the electron is excited to 5th energy level The total line in Paschen series and Balmer series are If it comes down by maximum transition 1 2 3 3 2 1 4 0

Physical Chemistry
Solid stateThe most unsymmetrical and symmetrical systems are respectively a Tetragonal Cubic b Triclinic Cubic c Rhombohedral Hexagonal d Orthorhombic Cubic The crystal system of a compound with unit cell paror

Physical Chemistry
Solid state4 A compound has BCC unit cell with edge length 10 if density is 2 g cm then molar mass of the compou is 1 240 3 350 2 300 4 280

Physical Chemistry
Solid stateTungsten has an atomic radius of 0 136 nm The density of tungsten is 19 4 g cm What is the crystal structure of tungsten Atomic weight W 184 a Simple cubic b Body centered cubic d None of these c Face centered cubic face centered cubic cell is 1 83 g cm at 20 C What is th

Physical Chemistry
Solid state17 An element having bcc geometry has atomic mass 50 Calculate the density of the unit cell if its edge length is 290 pm 1 6 81 g cm 3 13 62 g cm 2 3 40 g cm 4 1 23 q cm 3

Physical Chemistry
Solid stateThe edge length of face centred cubic unit cell having rock salt structure is 508 pm If the radius of the c is 110 pm the radius of the anion is 4 398 pm 1 144 pm 2 288 pm 3 618 pm

Physical Chemistry
Solid stateAssertion A particle present at the corner of the face centred unit cell has 1 8th of its contribution of the unit cell Reason In any space lattice the corner of the unit cell is always shared by the eight unit 2 B 3 C 4 D cells 1 A

Physical Chemistry
Solid state15 Consider a cube containing n unit cells of a cubic system A B a n 16 p Arrangement of atoms A plane ABCD obtained by joining the mid points of the edges on one of its identical faces had atoms arranged as shown Let p be the packing fraction Choose the correct option 22 21 2 11 21 D b n 8 p c n 4 p 14 B T

Physical Chemistry
Solid state23 In a simple cubic lattice of anions the side length of the unit cell is 2 88 The diameter of the void in the body centre is a 1 934 c 2 108 b 0 461 d 4 988

Physical Chemistry
Solid stateHow many unit cells are present in a cube shaped ideal crystal of NaCl of mass 1 00 g Atomic mass Na 23 C1 35 5 2 57 1021 B 128 10 1 A C D 5 14 1021 1 71x1021

Physical Chemistry
Solid state0 If the length of the unit cell is 54 the smallest 0 distance in between the two neighbouring A metal atoms in a face centred cubic lattice is 112 50 215 3 7 07 EAM 2011 4 3 535

Physical Chemistry
Solid state6 Body diagonal of a cube is 866 pm Its edge length would be 1 408 pm 3 500 pm 2 1000 pm 4 600 pm

Physical Chemistry
Solid stateA compound forms hexagonal close packed structure What is the total number of voids in 0 5 mol of it 0 1640 1 9 1023 3 3 10 3 2 6 10 3 4 1 8 10 3 ing blond

Physical Chemistry
Solid state44 The percentage of void space of a metallic element crystallising in a ABCABC type lattice pattern is 1 24 2 26 3 34 4 74

Physical Chemistry
Solid stateThe ratio of second coordination number of Nat in NaCl to first coordination number of Cst in CsCl will be 1 12 8 2 8 8 3 12 4 4 8 12

Physical Chemistry
Solid stateLithium has a BCC structure Its density is 530 kg m 3 and its atomic mass is 6 94 g mol Calculate the edge length of a unit cell of Lithium metal NA 6 02 x 1023 mol 2X6 94 1 154 pm 2 352 pm 3 527 pm 6 02 x 1023x 13 88 4 264 pm

Physical Chemistry
Solid state75 If metal m crystallises in bcc structure and has radius 1 86 A then the nearest neighbour distance and edge length will be respectively 1 3 72 A 5 26 A 3 72 3 72 4 3 A 2 0 93 5 26 0 93 A 4 3

Physical Chemistry
Solid stateS No Radius ratio 1 2 3 4 5 6 small large Geometric shape of the crystal formed upto 0 15 Linear 0 15 to 0 22 Trigonal planar 0 22 to 0 41 Tetrahedral 0 41 to 0 73 Square planar 0 41 to 0 73 Octahedral 0 73 to 0 99 Cubic Co ordi nation number of the ion 2 3 4 4 6 8

Physical Chemistry
Solid stateWhich is the incorrect statement a FeO0 98 has non stoichiometric deficiency defect b Density decreases in case of crystals with Schottky s defect c NaCl s is insulator silicon is semiconductor silver is conductor quartz is piezoelectric crystal d Frenkel defect is favoured in those ionic compounds in which sizes of cation and anions are almost equal NEET 2017 metal

Physical Chemistry
Solid stateThe number of octahedral void s per atom present in a cubic close packed structure is 1 2 3 1 2 4 4 3 AIPMT 2012

Physical Chemistry
Solid stateIn a face centered lattice with all the positions occupied by A atoms the body centered octahedral hole in it is occupied by an atom B of appropriate size For such a crystal calculate the void space per unit volume of unit cell Also predict the formula of the compound

Physical Chemistry
Solid state28 O In an ionic solid 1 6 A and Use the radius ratio rule to determine the edge length of the cubic unit 0 cell is A A 4 B 2 3 C 3 3 D 4 3

Physical Chemistry
Solid state25 AB is rock salt type crystal If radius of At is 75 pm then radius of B will be 1 181 2 pm 3 102 5 pm 2 333 3 pm 4 323 5 pm

Physical Chemistry
Solid stateThe flocculation value of HCl for arsenic sulphide sol is 30 m mol L If H SO4 is used for the flocculation of arsenic sulphide the amount in grams of H SO4 in 250 ml required for the above purpose is molecular mass of H SO4 98gm mol IPK of CH COOH 4 75

Physical Chemistry
Solid stateStructure of a mixed oxide is cubic close packed ccp The cubic unit cell of mixed oxide is composed of oxide ions One fourth of the tetrahedral voids are occupied by divalent metal A and the octahedral voids are occupied by a monovalent metal B The formula of the oxide is 1 ABO 3 A B O AIPMT Mains 2012 TV 2 Ov x 2 A BO 2 4 AB O 2

Physical Chemistry
Solid stateA C They melt over a range of temperature There is no orderly arrangement of particles B D They They are rigid and incom The number of hexagonal faces that are present in a truncated octahedron is 2 B 4 C 6 D 8 A The number of atoms contained in a fcc unit cell of a monoatomic substance is D 6

Physical Chemistry
Solid stateIf radius of an octahedral void is r and atomi radius of atoms assuming cubical close packing R Then the relation between r and R is 1 r 2R 2 r 1 414 R 3 r 0 414 R R 4 r