Solutions Questions and Answers

Physical Chemistry
SolutionsIE00 66 Which of the following has maximum solub K value is given in brackets 1 HgS 1 6 x 1054 3 ZnS 7 0 x 10 20 2 PbSO 1 3 x 1 4 AgCl 1 7 x 10 1500

Physical Chemistry
Solutions5 The solubility product constant K of Mg OH is 9 0x10 2 If a solution is 0 010 M with respect to Mg ion what is the maximum hydroxide ion concentration which could be present without causing the precipitation of Mg OH 1 1 5 x 10 M 3 1 5 x 10 M 2 3 0 10 M 4 3 0 x 105 M

Physical Chemistry
Solutions3 0 6 M 4 1 8 M The density of 3M solution of sodium chloride is 1 252 g mL The molality of the solution will be Molar mass NaCl 58 5 g mole 1 2 60 m 2 2 18 m 3 2 79 m 4 3 00 m

Physical Chemistry
Solutions1 Hydroxyl ion concentration OH in the case of sodium acetate can be expressed as where K is dissociation constant of CH COOH and C is the concentration of sodium acetate 1 OH CK K 2 2 OH C K K 3 4 OH M OH 1 2 C K 1 K C K K

Physical Chemistry
Solutions50 The expression of solubility product of mercurous iodide is 1 2 Hg x 2 1 1 2 3 Hg 1 1 1 2 Hg x 21 1 4 Hg2 12 1 12 150050 pr 1 3 59 O

Physical Chemistry
Solutions3 1 x 10 10 4 2 x 10 10 IE0065 64 56 The solubility product of As S is given by the expression 1 K As x S 2 K As 1 S 3 K As S22 4 K As 1 S4 150067 p

Physical Chemistry
Solutions12 litre of H and 11 2 litre of Cl are mixed and exploded The composition by volume of mixture is 1 24 litre of HCI 2 0 8 litre Cl and 20 8 lit HCL 3 0 8 litre H 22 4 litre HCI 4 22 4 litr HCL

Physical Chemistry
SolutionsA 0 2219 g sample of pure iron wire was dissolved in acid and the iron reduced to 2 state Then the solution required 34 65 mL of cerium IV in a titration Calculate the molar concentration of Ce solution A 0 1147 M B 0 1174 M C 0 1417 M D 0 1471 M

Physical Chemistry
SolutionsOf the following 0 10m aqueous solutions Q 2 which one will exhibit the largest freezing point depression 1 KCI 3 Al SO4 3 2 C6H12O6 4 K SOA A 0 10m 1 KCI 3 Al SO4 3 3 2 C H 2O6 4 K SO4

Physical Chemistry
Solutionsgain enthalpies of gaseous atoms must be determined in their respective most stable state i e ground state Q 3 15 Energy of an electron in the ground state of the hydrogen atom is 2 18 x 10 18 J Calculate the ionization enthalpy of atomic hydrogen in terms of kJ bold mol Ans Ionization energy is the amount of

Physical Chemistry
Solutions42 The vapour pressure of benzene at a certain 1 temperature is 640 mm of Hg A non volatile and non electrolyte solid weighing 2 175 g is added to 39 08 of benzene The vapour pressure of the solution is 600 mm of Hg What is the molecular weight of solid substance 1 69 45 3 49 50 2 59 6 4 79 8 PAD P P Conc AB 48

Physical Chemistry
Solutionsm s er e 5 At 100 C the vapour pressure of a solution of 6 5 g of a solute in 100 g water is 732 mm If K 0 52 the boiling point of this solution will be 1 69 A 1 103 C 3 100 C 2 101 C 4 102 C NEET 2016

Physical Chemistry
SolutionsGaseous benzene reacts with hydrogen gas in presence of a nickel catalyst to form gaseous cyclohexane according to the reaction C6H6 g 3H2 g C6H12 g A mixture of C6H and excess H has a pressure of 60 mm of Hg in an unknown volume After the gas had been passed over a nickel catalyst and all the benzene converted to cyclohexane the pressure of the gas was 30 mm of Hg in the same volume at the same temperature The fraction of C6H6 by volume present in the original volume is a 1 3 b 1 4 c 1 5 d 1 6

Physical Chemistry
Solutions30056 d earth 10 km speeds 62 In adjoining figure earth goes around the sun in elliptical orbit on which point the orbital speed is maximum 1 On A 2020 3 On C A D C

Physical Chemistry
Solutions7 Calculate the relative error in the concentration of the hydronium ion using concentrations instead of activities to calculate the concentration of the hydroxide ion OH in a NH30 0400 M in the presence of 0 100 M NaCl Your answer Typed answers are easier for studer
Physical Chemistry
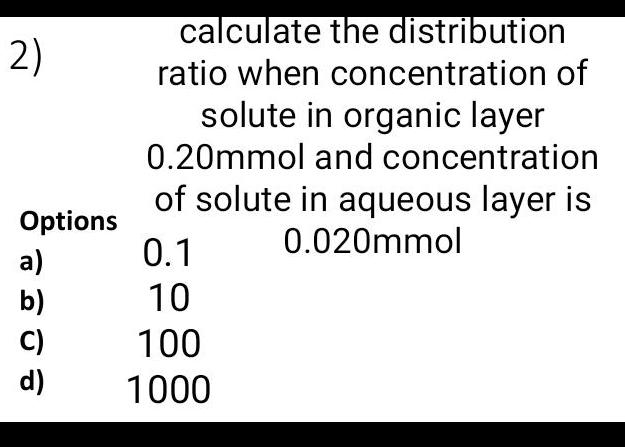
Solutions2 Options a b d calculate the distribution ratio when concentration of solute in organic layer 0 20mmol and concentration of solute in aqueous layer is 0 020mmol 0 1 10 100 1000

Physical Chemistry
SolutionsA solution of ethanol C H5OH in water has a concentration of 2 059 mol L 1 At 20 0 C its density is 0 9677 g mL 1 Calculate the following a the molality of the solution 2 36 m b the mass percent of the alcohol in the mixture

Physical Chemistry
SolutionsThe oxygen dissolved in water exerts a partial pressure of 20kPa in the mathon thons mathongo vapour above water The molar solubility of oxygen in water is mothongo mathongo mathongo x105 moldm 3 thongo Round off to the Nearest Integer Given Henry s law constant thango mathongemathongs mathongo mathango mathongo mathongs mathongo KH 8 0 x 10 kPa for O mathango mathongo mathongo mathongo mathongo mathongo mathongo mathongo mathongs mathongo

Physical Chemistry
SolutionsOn a vapour pressure versus temperature plot the curve of the frozen solvent meets the curve of the liquid solvent at a temperature A higher than that of the solution B lower than that of the solution C the same as that of the solution D which could be either higher or lower than that of the solution depending on the nature of the solvent

Physical Chemistry
Solutions3 The boiling point of 0 2 mol kg solution of X in water is greater than equimolal solution of Y in water Which one of the following statements is true in this case 1 Molecular mass of X is greater than the molecular mass of Y 2 Molecular mass of X is less than the molecular mass of Y ving that the moderator 3 Y is undergoing dissociation in water while X undergoes no change 4 X is undergoing dissociation in water while Y undergoes no change

Physical Chemistry
Solutionshave been denoted by and they upy part of the surface area area solven is correspondingly reduced thus the vapa 2 5 pressure is also reduced The decrease in the vapour pressure of solvent depends on t quantity of non volatile solute present in the solution irrespective its nature For example decrease in the vapour pressure of water adding 1 0 mol of sucrose to one kg of water is nearly similar to th produced by adding 1 0 mol of urea to the same quantity of water the same temperature A CRaoult s law in its general form can be stated as for any solution the partial vapour pressure of each volatile component in th solution is directly proportional to its mole fraction In a binary solution let us denote the solvent by Land solute AIDUAL CAMERA When the solute is non volatile only the solvent molecules present in vapour phase and contribute to vencur pressure Let p Shot by Sourddeep ar can you tell me vapour pressure of pure solvent without solute increases but non volatile solute is add v p is decrease then when volatile solute is add then what will 2021 08 26

Physical Chemistry
SolutionsTwo liquids a and b form an ideal solution such that p a is equal to 700 mm and p b is equal to 300mm a small amount of solution is vaporized and vapour condensed at equilibrium the conden sate has equilibrium vapour pressure of 500 mm at same temperature find the composition of bo of the original solution

Physical Chemistry
SolutionsExcess of NaOH aq was added to 100 mL of FeCl3 aq resulting into 2 14 g of Fe OH 3 The molarity of FeCl3 aq is Given molar mass of Fe 56 g mol and molar mass of Cl 35 5 g mol 1 0 3 M 2 0 2 M 3 0 6 M 4 18 M

Physical Chemistry
SolutionsT 12 The phase difference corresponding to path difference of x is 2x 1 2 2 x 3 4 WO0012 www com

Physical Chemistry
SolutionsWhat weight of pyrite ore should be taken for analysis so that the number of centigram of precipitated BaSO4 233 327 g mol will be twice the FeS2 119 847 g mol Please show complete solution with proper units and significant figures

Physical Chemistry
SolutionsThe molal depression constant for water 1 85 deg molal and for benzene is 5 12 deg molal If the ratio of the latent heats of fusion of benzene to water is 3 8 calculate the freezing point of benzene 1 5 75 C 2 5 12 C 3 4 97 C 4 6 1 C

Physical Chemistry
SolutionsA sample weighing 0 4564 g of a magnesium salt with 8 hydroxyquinoline gives a precipitate weighing 0 4717 g calculate the percentage of MgO in the sample assuming the precipitate is magnesium oxygenate dihydrate

Physical Chemistry
SolutionsAt 298 K 500cm H O dissolved 15 30 cm CH4 STP under a partial pressure of methane of one atm If Henery s law holds what pressure is required to cause 0 001 mole methane to dissolve in 300cm water 1 0 286 atm 2 2 486 atm 3 1 286 atm 4 3 111 atm

Physical Chemistry
SolutionsTypes of Solution There are some of the characteristics of the supersaturated solution I Equilibrium exists between solutions and solid solute II If a crystal of solute is added to supersaturated solution crystallisation occurs rapidly III Supersaturated solutions contain more solute 5 than they should have at a particular temperature Correct characteristics of supersaturated solutions are A I II III C I III B II III D I II 4 Octane non pc Which pair of liqui A Water and oct B Water and hex C Hexanol and D Chloroform a An ionic compoun so strongly that a A Dilute C Immiscible S

Physical Chemistry
SolutionsAmount of heat evolved when 500 cm of 0 2 M H SO4 is mixed with 200 cm of 0 5 M NaOH solution is 11 4 kJ 2 85 kJ 5 71 kJ 28 5 kJ Solution Answer 3 mol of H SO4 500 x 0 2 1000 mol of H 2 x 0 1 0 2 mol 200 x 0 5 mol of NaOH 1000 So amount of heat evolved 57 1 x 0 1 0 1 mol 0 1 mol mol of OH

Physical Chemistry
Solutions3 Hg 1 60 At 25 C the K value of AgCl is 1 8 x 10 10 IE 10 5 moles of Ag are added to solution then Kop sp wil sp be 1 1 8 x 10 15 3 1 8 x 10 5 2 1 8 10 10 4 18 x 10 10 tor to dissolve 1

Physical Chemistry
SolutionsNone 127 What is the quantity of NaOH present in 250 cc of the solution so that it gives a pH 13 1 10 13 g 2 10 g 3 1 0 g 4 4 0 g 128 0 001 mol of the strong electrolyte M OH has

Physical Chemistry
Solutions169 Two containers A and B contain molecular gas at same temperature with masses of molecules are m and m then relation of momentum P and P will be 1 P PB 2 P 3 P 4 P MA ma mg MA 1 2 1 2 PB PB m P B ma 174 175

Physical Chemistry
SolutionsMolar ratio of CO CH4 O2 in a gas sample over water at 25 C are 1 5 40 assuming Henry s constant value for CO CH4 O2 are 1 67 0 41 34 86 then order of the solubility are CD CF 0 CH 0 00

Physical Chemistry
Solutions1 The equilibrium constant K for the thermal dissociation of 12g intolat 200 C is 4 0 atm The pressure at which is 50 dissociation at the same temperature 1 2 0 atm 2 3 0 atm 3 4 0 atm I 2 I g C X 2x MT C x 3x1 Kp etx

Physical Chemistry
SolutionsWhat is the mass of the precipit 50 mL of 16 9 w v solution of AgNO3 is mixed with 50 mL of 5 8 w v NaCl solution Ag 107 8 N 14 O 16 Na 23 CI 35 5 Re AIPMT 2015 1 7g 2 14 g

Physical Chemistry
SolutionsThe boiling point of a solution of 5 g of sulphur in 100 g of carbon disulphide is 0 474 C above that of pure solvent Determine the molecular formula of sulphur in this solvent The boiling point of pure carbon disulphide is 47 C and its heat of vaporisation is 84 calories per gram RT 2 22012

Physical Chemistry
Solutionscases T0147 ressure ill b 141 Simple behaviour under all conditions of real gas is governed by the equation 1 PV RT sion 20 2 P V b RT 3 PV 4 PV constant constant

Physical Chemistry
SolutionsA non volatile solute P is present in 500 mL solution and molarity is found to be 1 M A sample of 5 mL of this solution is taken in another container and further 0 4 g of P is added and volume is increased by adding water such that final volume becomes 10 L If molarity of diluted solution is 10 M the molar mass of solute P in g mol is A solur

Physical Chemistry
SolutionsA 300K 400K Vapour pressure of liquid at 300 K is 20 mm of Hg and 400 K is 40 mm of Hg Very long time after opening the stopper the total pressure will be Question Type Single Correct Type 120 mm of Hg 2 40 mm of Hg 3 B 30 mm of Hg

Physical Chemistry
SolutionsIn a binary solution of methyl alcohol and water if the masses of methyl alcohol and water are 48 g and 81 g respectively then the mole fraction of methyl alcohol in the solution is O 0 5 O 0 25 0 33 O 0 15

Physical Chemistry
Solutions83 Velocity time curve for a body projected upwards is a an 1 Parabola 2 Ellipse 3 Hyperbola

Physical Chemistry
Solutions5 When a ball is thrown vertically up with velocity v it reaches a maximum height h If one wishes to triple the maximum height then the ball should be thrown with velocity 1 3 2 3v 3 9V asion

Physical Chemistry
Solutions24 73 For A g B g C g reaction the differential rate d A K A dt Initially the pressure is 100 mm of Hg after 10 minutes total pressure is 120 mm of Hg Hence rate constant min is law expression is given as r 1 k 3 k 2 303 120 log 10 100 2 303 10 100 80 log 2 k 4 k 2 303 100 log 10 20 100 120 CK0222 2 303 10 log

Physical Chemistry
Solutions100 mL of an aspirin solution is diluted to 250 mL and titrated with 0 1106 M NaOH The equivalence point is obtained by adding 9 62 mL of NaOH Determine the pH of the original aspirin solution Ka 1 75 x 10 5

Physical Chemistry
SolutionsWhich of the following solutions will have highest osmotic pressure A B D 1 Glucose in water 1 Sucrose in water 1 CaCl in water None of these

Physical Chemistry
Solutions18 gm of glucose is dissolved in 100 gm of water Calculate the difference between B pt F pt of solution K C K m and Kf 1 86 K m A B 102 38 98 66 4 00 1 34

Physical Chemistry
SolutionsHenry s law constants for four gases are given below at 293 K at constant pressure Which one is less soluble in water that temperature A B D A KH 34 86 Kbar B KH 69 16 Kbar C KH 144 97 Kbar D KH 88 84 Kbar

Physical Chemistry
Solutionsto have a pH of 4 5 The ionisation constant K for hydrazine NH is 1 0 x 10 6 What would be the age hydrolysis of 0 001 M N H CT a salt containing acid ion conjugate to hydrazine base 2 0 x 10 5 A 0 25 M solution of pyridinium chloride C H NHCl was found to have a pH of 2 89 What is the pK for nyriding CHN

Physical Chemistry
Solutions87g K SO4 At wt of K 39 is dissolve in 500g of H O then determine freezing point and boiling point of solutie Given K H 0 1 8 Kkgmol 0 5 Kkgmol Kb H 0 A B 5 4 C 101 5 C 5 4 C 100 5 C 5 4 C 101 5 C 1 8 C 100 5 C