Solutions Questions and Answers

Physical Chemistry
Solutions37 2 points What is the solvent in a solution of 2 cycle engine mix consisting of 19 L of gasoline and 600 mL of oil Gasoline Oil Water Previous

Physical Chemistry
Solutions35 2 points As the intermolecular forces increase the vapor pressure of a liquid decreases 00 True False Previous

Physical Chemistry
Solutions12 3 4 23 2 points In a mixture of gases the gas that is present in the greatest amount is called the solvent solution solute Previous

Physical Chemistry
Solutions1 2 points What is the solvent in supersaturated sodium acetate solution containing 90 g of sodium acetate and 10 g of water Sodium Water Sodium acetate

Physical Chemistry
Solutionsculate the molecular weight of an unknown nonionic solute if 5 78 g are dissolved in 525 mL tion and the osmotic pressure is 47 torr at 30 C x unit g mol 4E 5 stion Help Written Example ubmit Question OF

Physical Chemistry
Solutions10 2 points A saline solution contains 0 80 w v of NaCl What is the molarity of the solution O 0 154 1 29 0 137 O 0 129

Physical Chemistry
Solutions47 2 points In a pressure cooker at 1 2 atm the boiling point of water will remain unchanged increase decrease Previous

Physical Chemistry
Solutions2 points What is the solvent in a solution of one mole of O gas mixed with one half of a mole of O3 gas O 02 03 Water Previous


Physical Chemistry
SolutionsA cell with a K concentration of 0 3 eq L and a Na concentration of 0 1 mol L is placed into a solution with a K concentration of 0 2 eq L and a Na concentration of 0 2 mol L It is assumed that the K and Na can both pass through the cell membrane the K and Na transport proteins are open Answer the following true or false questions a true b false Diffusion of a solute through the membrane is called dialysis K1 will dialyze diffuse out of the cell Na will dialyze diffuse into the cell Like the diffusion of gases diffusion of particles in a liquid is independent of the other solutes

Physical Chemistry
SolutionsZn s PbCl2 aq ZnCl aq Pb s If 25 0 g of zinc metal are reacted with 30 0 g of lead II chloride zinc chloride and lead metal are formed Calculate the mass of zinc chloride produced 16 7 g ZnCl 14 7 g ZnCl 17 4 g ZnCl 11 7 g ZnCl

Physical Chemistry
Solutions1 point How many moles of chlorine gas are needed to react with 3 20 mols of sodium to produce sodium chloride Cl g Na s NaCl s unbalanced 1 90 mol Cl 2 60 mol Cl 1 60 mol Cl 1 80 mol Cl

Physical Chemistry
Solutions3 If 14 45 ml of H SO4 aq is required to reach the end point when titrated with 25 00 mL of your standardized sodium hydroxide solution use your average molarity what is the concentration of the acid Write the balanced chemical equation for this neutralization reaction

Physical Chemistry
SolutionsA student is making a solution of sugar in water If the student uses 0 83 moles of sugar and enough water to make 529 83 milliliters of solution what is the molarity of the student s sugar solution Round your answer to the nearest 0 01 and include units properly abbreviated but NOT substance Your Answer

Physical Chemistry
Solutions11 You are trying to do an experiment that calls for 0 50 L of 0 800 M HF solution but you only have concentrated 10 0 M solution in your stock room How much of the concentrated solution will you put in your volumetric flask before diluting to 0 50 L with water

Physical Chemistry
Solutionsf the surface area of a solute is decreased its solubility decreases O increases O is not affected O first increases and then decreases

Physical Chemistry
SolutionsWhich of the following factors affects the solubility of a solute in a solvent O Temperature Pressure O Surface area of the solute substance All of the choices

Physical Chemistry
SolutionsWhich of the following will NOT directly affect the solubility of a substance Surface area Pressure Witnessing Temperature

Physical Chemistry
SolutionsWhen a solid dissolves in water heat may be evolved or absorbed The heat of dissolution dissolving can be determined using a coffee cup calorimeter In the laboratory a general chemistry student finds that when 1 41 g of CaCl s are dissolved in 114 80 g of water the temperatur of the solution increases from 24 47 to 26 70 C The heat capacity of the calorimeter sometimes referred to as the calorimeter constant was determined in a separate experiment to be 1 59 J C Based on the student s observation calculate the enthalpy of dissolution of CaCl s in kJ mol Assume the specific heat of the solution is equal to the specific heat of water AH dissolution kJ mol

Physical Chemistry
Solutions5 844 g of sodium chloride is added to 1000 mL of water at 34 C How will this affect the freezing point of water Sodium chloride is an electrolyte and the density of water at 34 C is 0 994 g mL The freezing point depression constant K of water is 1 86 C kg mol Molecular mass of sodium chloride is 58 44

Physical Chemistry
SolutionsConceming the solvation step of the solution formation process O the process is endothermic and the potential energy decreases O the process is exothermic and the potential energy increases the process is endothermic and the potential energy increases the process is exothermic and the potential energy decreases O the process is endothermic and occurs with no change in potential energy

Physical Chemistry
Solutions3 A student titrates 25 0 mL of HCI using a standard solution of 0 250 M NaOH What is the molarity of the HCI solution if it takes 35 0 mL of NaOH to reach the titration end point What is the mass of HCI in the sample used

Physical Chemistry
SolutionsWhat is the pH of an aqueous solution of 6 83x10 2 M potassium hydroxide pH

Physical Chemistry
SolutionsChemical Calculations Give the answer in the available space 53 61 mL of acetic acid concentration 0 898 mol L gets titrated with 43 71 mL sodium hydroxide What is the concentration of the sodium hydroxide solution Give the answer in 3 decimal places

Physical Chemistry
SolutionsHow many grams do you need to dissolve the make the following solution 250 0 mL of 9 7 M ammonium sulfate NH4 2SO4 V


Physical Chemistry
SolutionsYou have standardized a solution of HCL with a nominal concentration of 6 moles per liter and found the true concentration to be 6 083 moles liter You need to make 1 liter diluted solution as accurately as possible How many mL of this standardized solution do you need to add to a class A volumetric flask to make a 0 1 molar solution in HCL 2 points

Physical Chemistry
SolutionsA B What is the freezing point when the percent weight of ethylene glycol is 55 Assume the data is linear 5 39 C 40 C 41 C Ethylene glycol Concentration in water vs freezing boiling point 44 C Weight Freezing Boiling Point C Point C EG 0 10 20 30 40 50 60 70 80 90 0 4 7 15 23 34 48 51 45 29 100 102 102 104 104 107 110 116 124 140

Physical Chemistry
SolutionsThe supercript o in AH refers to O 0 C and 0 atm O 273 C and 760 mmHg O 273 K and 1 atm O 298 K and 1 atm O 298 K and 760 atm

Physical Chemistry
SolutionsO 790 mL O240 mL 83 mL 430 mL O 520 mL 310 mL O Not enough information to answer Question 7 2 A chemist has a 0 500 M solution of potassium hydroxide molar mass 56 1 mol What mass of KOH is dissolved in 2 70 L of the solution

Physical Chemistry
Solutionstermine the molarity of a solution with a volume of 622 mL and 0 730 mol of solute dissc M swer

Physical Chemistry
SolutionsA sample of oxygen gas was collected by displacement of water in a gas collection apparatus The total pressure in the collection vessel was 728 torr the temperature was 26 0 C and the vessel contained 522 mL of the collected gas At 26 0 C the vapor pressure of water is 25 6 torr What is the partial pressure in torr of oxygen gas Keep the answer with 1 decimal place Do not write unit in answer

Physical Chemistry
SolutionsHow many meq of PO4 are there in 50 0 mL of a 0 250 M Mg3 PO4 2 Solution The reaction is given so you can get the conversion factor Milliequivalent i abbreviated as meq 4 Mg3 PO4 2 OA 0 0750 meq OB 12 5 meq O C 37 5 meq D 25 0 meq 3 Mg 2 2 PO4 E 75 0 meq

Physical Chemistry
Solutions9 1 point Dilute hydrochloric acid has a concentration of 6 0 mole L What is the w v percent concentration 0 219 0 213 21 9 O 21 3

Physical Chemistry
Solutions0 4 osM wwwwwww 0 3 OSM wwwwwww True or false about the diagram where a cell is placed into the 0 4 osM solution the process is called dialysis True False Reset Selection

Physical Chemistry
Solutions33 4 points Nitrogen gas N has a solubility in water of approximately 0 0167 g L at 25 0 C and 1 00 atm What is the solubility g L of N in water in Denver where the atmospheric pressure is approximately 0 913 atm Do not report units in your answer Report your answer with 4 places past the decimal point uropower

Physical Chemistry
SolutionsNitrogen gas N has a solubility in water of approximately 0 0173 g L at 25 0 C and 1 01 atm What is the solubility g L of N in water in Denver where the atmosphe pressure is approximately 0 893 atm Do not report units in your answer Report your answer with 4 places past the decimal point Type your answer

Physical Chemistry
Solutions10 6 Calculate the pH for the solutions which have the following OH 9 5 x 10 6 7 9 x 10 3 4 4 x 10 10

Physical Chemistry
SolutionsTums calcium carbonate is an ant 1p acid It neutralizes stomach acid Its pH would most likely be less than 7 O exactly 7 greater than 7

Physical Chemistry
SolutionsMethane CH4 burns in air by the following reaction CH4 2 O2 CO 2 H O What mass of water is produced by burning 500 0 grams of CH4

Physical Chemistry
SolutionsDetermine if H O is a suitable reagent to protonate each of the two compounds shown below CH3 O S O HO S O I OI yes Il no OI no Il no O I no Il yes Ol yes Il yes NH

Physical Chemistry
SolutionsA solution has OH 2 3 x 10 7 M The H in this solution is O 1 0 M O 2 3 107 M 1 0 10 7 M O 4 3 x 10 8 M O none of these

Physical Chemistry
SolutionsRead the chemical equation Fe2O3 CO Fe CO If 2 moles of Fe O3 react with 9 moles of CO how many moles of each product are formed O 1 mole Fe and 2 moles CO2 O 2 moles Fe and 3 moles CO 4 moles Fe and 6 moles CO2 O 6 moles Fe and 9 moles CO2

Physical Chemistry
SolutionsIf sodium sulfate reacts with 20 grams of hydrochloric acid how many grams of sulfuric acid will be produced Na SO 2HCl 2 NaCl H 304

Physical Chemistry
SolutionsHow many moles of solute are present in 560 mL of a 5 1 M solution Round your answer to the nearest 0 01 and remember to include properly abbreviated units

Physical Chemistry
SolutionsFor compound A AH vap 30 91 kJ mol and AS vap 93 23 J K mol at 25 C What is the normal b equilibrium for compound A 25 K 332 K 58 K

Physical Chemistry
SolutionsIf 0 0742 mol CaCl is dissolved in water to make a 0 270 M solution what is the volume of the solution
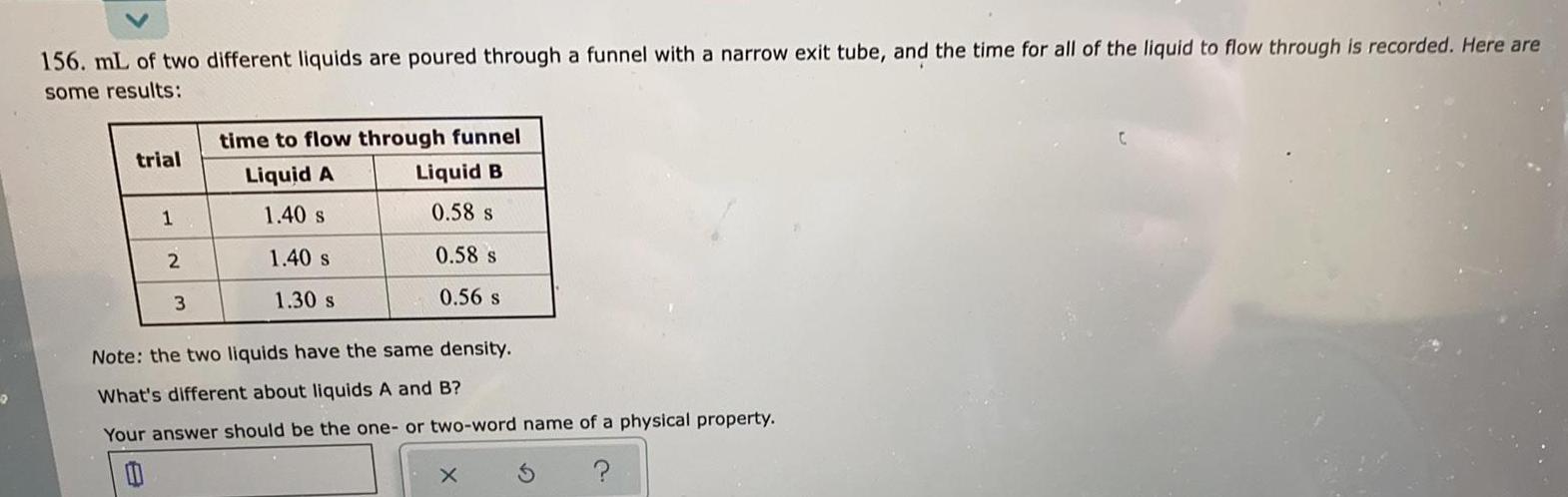
Physical Chemistry
Solutions2 156 mL of two different liquids are poured through a funnel with a narrow exit tube and the time for all of the liquid to flow through is recorded Here are some results trial 1 2 3 time to flow through funnel Liquid A Liquid B 1 40 s 0 58 s 1 40 s 1 30 s 0 58 s 0 56 s Note the two liquids have the same density What s different about liquids A and B Your answer should be the one or two word name of a physical property 0 S X

Physical Chemistry
SolutionsViscosity is resistance to flow Liquids that flow slowly and are hard to stir or pour have a high viscosity Liquids that flow easily and quickly have a low viscosity For example honey has a high viscosity and water has a low viscosity Viscosity also controls how fast objects move through liquids the higher the viscosity the harder it is for objects to push through the liquid Surface tension is resistance to penetration of a surface or any increase in surface area Fluids that form more compact rounder drops on surfaces or which more strongly resist penetration by small objects have a high surface tension Vapor pressure is how quickly and completely a liquid evaporates Liquids with a high vapor pressure evaporate quickly and when in a closed container produce a higher partial pressure of vapor at equilibrium All three of these properties are the result of intermolecular forces between the atoms or molecules that make up the liquid In this case both drops have the same curvature so the surface tension of the two liquids must be the same However the drop of Liquid X is evaporating faster because it gets smaller faster That means Liquid X has a higher vapor pressure Liquid Y EO ANSWER vapor pressure

Physical Chemistry
Solutionsthe absolute temperature and the gas constant R Suppose the osmotic he osmotic pressure exerted by a solution is equal to the molarity multiplied pressure of a certain solution is measured to be 14 atm at an absolute temperature of 376 K Write an equation that will let you calculate the molarity c of this solution Your equation should contain only symbols Be sure you define each symbol other than R Your equation C Definitions of your symbols 14 atm 376 K E F A