Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
GeneralGold ornaments are made by alloying Au with Cu An ornament contains copper and gold in equimolar ratio in it Weight percentage of gold in the ornament is To the nearest integer Atomic mass of gold 197 and of copper 63 5 amu

Physical Chemistry
Atomic StructureWhich of the following information is not correct about principal quantum number n 1 As value of n increases the energy of the orbit increases 2 As value of n increses the distance from the nucleus increases 3 As value of n increases the velocity of electron increases 4 The maximum number of electrons in a particular shell is equal to 2n

Physical Chemistry
Gaseous and liquid statesQuestion No 37 In the van der Waals gas equation the term which indicates the volume occupied by the gas molecules is V nb O O O an v nb an P a

Physical Chemistry
General18 Inert gas has been added to the following equilibrium system at constant volume SO g 1 2 0 g 0 g To which direction will the equilibrium shift a Forward b Backward c No effect d Unpredictable

Physical Chemistry
EnergeticsKoq gener A aq The AG of the reaction is 9 770 kJ mol Calculate the equilibrium constant for the reaction at 25 C enzyme AG B aq What is AG for the reaction at body temperature 37 0 C if the concentration of A is 1 8 M and the concentration of B is 0 45 M kJ mol

Physical Chemistry
Gaseous and liquid states11 Assertion Most probable velocity is the velocity possessed by maximum fraction of molecules at the same temperature Reason On collision more and more molecules acquire higher velocity at the same temperature

Physical Chemistry
General2 Which of the following metal sulphide has maximum solubility in water a CdS K 36 10 sp b FeS K 11 10 2 sp c HgS K 32 10 54 sp d ZnS K 11 x 10 sp

Physical Chemistry
GeneralIron forms a sulphide with formula Fe7Sg Iron exist in both 2 and 3 oxidation states The ratio of Fe II atoms to Fe III atoms is Question Type Single Correct Type 1 2 3 3 4 4 3 2 5

Physical Chemistry
Solutions0 A gaseous mixture contains equal masses of O2 and SO2 The molar ratio of the two gases in the mixture is 1 2 3 2 2 1 3 4 1 4 1 4 1 Mole fraction of the solute in a 2 molal aqueous solution is 1 0 17 3 0 035 2 0 017 4 0 35

Physical Chemistry
GeneralA colloid prepared by the addition of KI to AgNO solution is purified using dialysis Find the minimum mass in grams of an electrolyte AB GMM 60 required to completely coagulate 1 of the aforementioned colloid Given Active ion causing flocculation A B Flocculation value mmol l 50 100

Physical Chemistry
Atomic Structure68 The wavenumber of a radiation whose frequency is 7 5 10 s 1 is 1 1 25 m 1 2 0 25 m 1 3 2 5 m 1 4 25 m 1 69 The species which has the maximum charge to mass ratio is 1 Electron 3 Neutron 2 Proton 4 a particle

Physical Chemistry
GeneralIf pressure and temperature are kept constant the reaction of 18 mL of N gas with 54 mL of H gas will form Select one O a 27 Ob 9 c 108 O d 18 e 36 ml of ammonia

Physical Chemistry
Gaseous and liquid statesQuestion No 36 Pressure exerted by 16 g of oxygen gas in 0 01 m vessel at 47 C is O 4 5 atm O 1 3 atm 0 21 atm 6 8 atm

Physical Chemistry
General5 moles of nitrogen gas are enclosed in an adiabatic cylindrical vessel The piston itself is a rigid light cylindrical container containing 3 moles of Helium gas There is a heater which gives out a power 100 cal to the nitrogen gas A power of 30 cal is transferred to Helium through the bottom surface of the piston The rate of increment of temperature of the nitrogen gas assuming that the piston moves slowly He N 1 2K sec 2 4K sec 3 6K sec 4 8K sec

Physical Chemistry
GeneralA freshly prepared Fe OH 3 precipitate is peptized by adding FeCl3 solution The charge on the colloidal particle is due to preferential adsorption of Question Type Single Correct Type 1 CI ions 2 Fe ions 3 OH ions

Physical Chemistry
Chemical BondingConsider the following statements a NO is an odd electron molecule b 03 molecule has two resonating structures c PF5 follows octet rule The correct statements is are O a and b only Ob and c only O a and c only a b and c

Physical Chemistry
Equilibriumc Between 8 and 9 d Between 6 and 7 8 The pH of a dilute solution of acetic acid was found to be 4 3 The addition of a small crystal of sodium acetate will cause pH to a Become less than 4 3 b Become more than 4 3 c Remains equal to 4 3 d Unpredictable

Physical Chemistry
GeneralTwo liquids A and B have vapour pressure in the ratio P Pg 1 3 at a certain temperature Assume A and B form an ideal solution and the ratio of mole fractions of A to B in the vapour phase is 4 3 Then the mole fraction of B in the solution at the same temperature is 1 2 3 4 1 5 5 1

Physical Chemistry
Generald 8 5 33 A g B g AB g is a reversible reaction At equilibrium 0 4 mole of AB is formed when each A and B are taken one mole How much of A changes into AB a 20 c 60 b 40 d 4

Physical Chemistry
Atomic Structure2 Energy and size of the shell is determined by 1 Principal Quantum Number 2 Azimuthal Quantum Number 3 Magnetic Quantum Number 4 Spin Quantum Number 3 The ratio of the wavelength of the first line of Balmer series to that of the first line of Paschen series of hydrogen atom is 1 7 20 2 20 7 3 25 9 4 8 27

Physical Chemistry
Atomic StructureWhich of the following statements is not correct regarding to galvanic cell Question Type Single Correct Type 1 Cell reaction is spontaneous from left to right if Ecell 0 2 Cell reaction occurs from right to left if Ecell 0 If the system is at equilibrium no net reaction 3 occurs 4 Ecell is temperature independent

Physical Chemistry
Atomic StructureThe orbital angular momentum of an electron present in 3p orbital is 1 2h 2 Zero 3 6 h 4 2 2h Maximum number of orbital s in an atom that have quantum number n 4 1 1 m 1 is 1 4 3 2 2 3 4 1

Physical Chemistry
Energeticsf 10 Assertion The heat absorbed during the isothermal expansion of an ideal gas against vacuum is zero Reason The volume occupied by the molecules of an ideal gas is zero

Physical Chemistry
Generalc 10 3 d 10 2 45 The dissociation constant value of four acids are as follows Which of the values stand for strongest acid a 2x 10 4 b 2 x 10 c 3 10 4 d 0 02 10 46 Strongest lewis acid among the following in

Physical Chemistry
Atomic StructureTest 01 Code A 84 In which of the following orbital diagrams Pauli s exclusion principle Hund s rule of maximum multiplicity and Aufbau principle all are violated 3s 1 1 2 1 3 1 4 1 85 S is isoelectronic with 1 Ca 3 Na 3p 11 1 1 1 111 1 1 1 2 K 4 Mg2

Physical Chemistry
EquilibriumThe molar ratio of sodium acetate to acetic acid in a buffer solution with a pH of 5 76 is 5 1 Assuming the total buffer concentration is 2 2 x 102 mol L how many grams of sodium acetate m w 82 should be used in preparing a liter of the solution Round answer to the nearest hundredth Do not include units

Physical Chemistry
GeneralTwo mole of an ideal gas is expanded isothermally and reversibly from 1 litre ot 10 litre at 300 K The enthalpy change in kJ for the process is IIT JEE Screening 2004 1 11 4 kJ 2 11 4 kJ 3 0 kJ 4 4 8 kJ

Physical Chemistry
General44 4 4 g of an ideal gas mol wt 44 at 300K and 10 atm is allowed is allowed to expand isothermally against a external pressure of 1 atm Taking 1 L atm 100 J heat absorbed by the gas is a 200 5 J b 212 5 J c 221 4 J d 242 4 J

Physical Chemistry
SolutionsIn a 0 2 molal aqueous solution of a weak acid HX the degree of ionization is 0 3 The freezing point of the solution is nearest to Kf of water 1 85 Question Type Single Correct Type 1 0 360 C 20 480 C 3 0 260 C

Physical Chemistry
GeneralFor which one of the following is AH re reaction equal to AH f for the product Question Type Single Correct Type 1 2 3 4 2CO g 02 g 2CO2 g N2 g 03 g N203 g CH4 g 2Cl2 g CH Cl2 e 2HCl g Xe g 2F2 g XeF4 s

Physical Chemistry
Surface chemistry1 Ethyl chloride 2 Isopropyl chloride 3 CI On of the processes used for concentration of ores is Froth floatation process This process is generally used for concentration of sulphide ores Sometimes in this process we add NaCN as a depressant NaCN is generally added in case of ZnS and PbS minerals What is the purpose of addition of NaCN during the process of Forth floatation 1 NaCN causes reduction by precipitation 2 A soluble complex is formed by reaction between NaCN and ZnS while PbS forms froth 3 A soluble complex is formed by reaction between NaCN and PbS while ZnS forms froth 4 A precipitate of Pb CN is produced while ZnS remain unaffected Which fah

Physical Chemistry
Electrochemistry1 Identify the complete redox reaction for a Zn Zn2 Cu Cu cell A Zn s Cu aq Zn aq Cu s Zn aq Cu aq Zn s Cu aq Zn aq 2 Cu aq B Zn s Cu s C Zn aq Cu s D Zn s 2 Cu s s 2 Identify the complete redox reaction for a Zn Zn Pb2 Pb cell aq Pb aq Zn s Pb aq Zn aq 2 Pb s 7 21 Phal A Zn s Pb s Zn B Zn aq Pb s C Zn s 2 Pb aq 2 Choose Choose

Physical Chemistry
General7 If 0 3 mol of BaCl2 is mixed with 0 1 mol of Na2SO4 then the mass of precipitate formed is Atomic mass of Ba is 137 u 1 36 2 g 2 42 1 g 3 46 6 g 4 23 3 g 3 The weight of the residue obtained on strongly heating 21 g of MgCO3 is 1 10 g 3 7 g 2 12 g 4 15 g

Physical Chemistry
ElectrochemistryWhen 1 mole of an ideal gas at 20 atm pressure and 15 L volume expands such that the final pressure becomes 10 atm and the final volume become 60 L Calculate entropy change for the process Cpm 30 96 J mole 1 K 1 80 2 J k Imo1 1 2 62 42 kJ K 1 mol 1 3 120 x 102 J1c mol 1

Physical Chemistry
Chemical BondingIn which of the following pairs hydrogen bond is present O He and Cl O HCI and H O NH3 and H O O H O and H
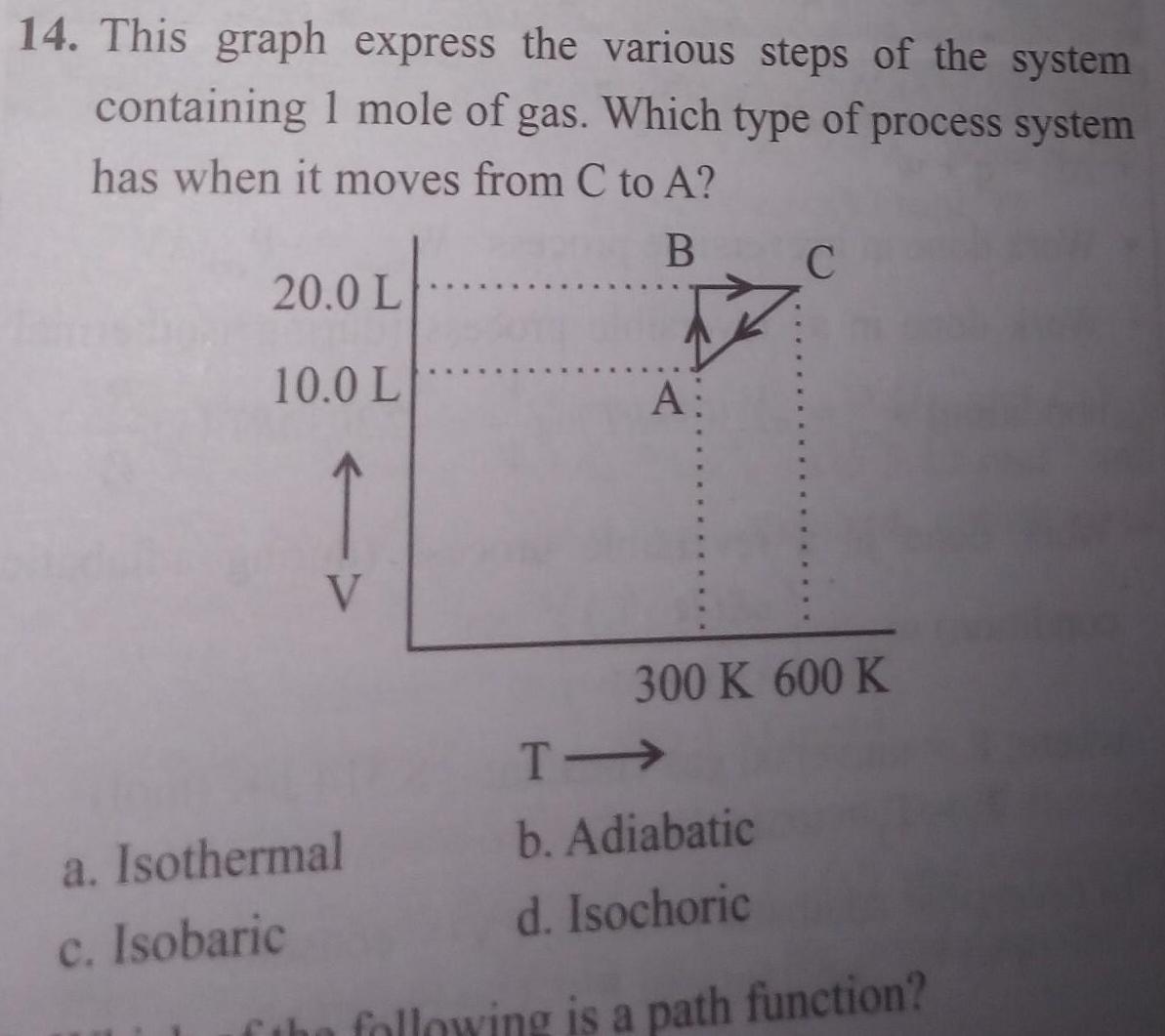
Physical Chemistry
Gaseous and liquid states14 This graph express the various steps of the system containing 1 mole of gas Which type of process system has when it moves from C to A B 20 0 L 10 0 L V A a Isothermal c Isobaric C 300 K 600 K T b Adiabatic d Isochoric the following is a path function

Physical Chemistry
Solutions3 The chemical potential of pure benzene decreases by 250 J when an amount of paraffin wax is dissolved in it at 25 C and 1 atm At what temperature does this solution boil M Benzene 78 11 g mol Boiling point of pure benzene 80 1 C Boiling point constant of benzene K 2 53 K kg mol

Physical Chemistry
Equilibrium15 At 600 C K for the following reaction is 1 atm P X g Y g Z g at equilibrium 50 of X g is dissociated The total pressure of the equilibrium system is P atm What is the partial pressure in atm of X g at equilibrium a 1 b 4 c 2 d 0 5

Physical Chemistry
Gaseous and liquid states31 At constant temperature the equilibrium constant K for the decomposition reaction N O g 2NO is expressed by 4 K P 4x P 1 x where P pressure x extent of decomposition Which of the following statements is true a K increases with increase of P P b K increases with increase of x P c K increase with decrease of x P d K remains constant with change in P or Y

Physical Chemistry
GeneralQuestion 9 The digestive system is made up of the gastrointestinal tract also called the GI tract or digestive tract and the liver pancreas and gallbladder The GI tract is a series of hollow organs joined in a long twisting tube from the mouth to the anus The food and liquids pass through these organs during their processing and get converted into forms absorbable into the bloodstream i Digestion of food starts from which organ of the digestive system a Mouth due to the presence of saliva b Oesophagus that moves the food towards stomach c Small intestine that releases juices for fat breakdown d Stomach which helps in churning the food ii A student sets up an experiment to study the role of enzymes in digestion of food In which test tube the digestion of protein will occur a Test tube A as pepsin will breakdown into simple molecules b Test tube B as HCI will breakdown protein into simple molecules c Test tubes A as pepsin will breakdown protein into simple molecules d Test tube B as HCI will activate pepsin for breakdown of protein into simple molecules A Test tube Egg white Pepsin B Egg white Pepsin HCI iii Why is the inner wall of the alimentary canal not digested although the dige enzymes can digest all the materials that make cells 3 0

Physical Chemistry
Energeticshydration 8 The heat of combustion of benzene determined in a bomb calorimeter is 870 k Cal mol at 298 K The value of AE for the reaction is a 1740 k Cal mol b 870 k Cal mol c 870k Cal mol d 1740 k Cal mol

Physical Chemistry
GeneralA sample of 5 litre gas in open vessel is taken at 300 K temperature and it is heated upto 450 K then what fraction of gas will escape out with respect to final volume 1 50 3 33 33 2 25 4 100

Physical Chemistry
Gaseous and liquid states28 The work done on the system when one mole of an ideal gas at 500 K is compressed isothermally and reversibly to 1 10th of its original volume R 2 cal a 500 kCal b 1 51 kCal c 23 03 kCal d 2 303 kCal

Physical Chemistry
Gaseous and liquid statesA gas at 350 K and 15 bar has molar volume 20 percent smaller than that for an ideal gas under the same conditions The correct option about the gas and its compressibility factor Z is NEET 2019 1 Z 1 and attractive forces are dominant 2 Z 1 and repulsive forces are dominant 3 Z 1 and attractive forces are dominant 4 Z 1 and repulsive forces are dominant

Physical Chemistry
GeneralConsider the zinc blende structure formed by the sulphide ions occupying the FCC lattice points and zinc ions occupying some of the tetrahedral voids Select the correct statement about the crystal assuming it to be ideal The coordination number of Zn and S is same and equal to 4 The contribution of the S ions towards the packing efficiency is 74 approx The contribution of the Zn ions towards the packing efficiency is 1 approx The packing efficiency of the ZnS structure is 78 9 approx

Physical Chemistry
EnergeticsOne mole of a real gas is subjected to heating at constant volume from P V T state to P2 V2 T state Then it is subjected to irreversible adiabatic compression against constant external pressure of P3 atm till syatem reaches the final state P3 V3 T3 If the constant volume molar heat capacity of real gas is Cy Find out correct expression for AH from state 1 to state 3 1 C T3 T P3V P V 2 Cv T2 T1 P3V2 P V 3 Cv T2 T1 P3V P V 4 Cp T2 T1 P3V P V

Physical Chemistry
Generalc 44 g Match the mass of elements given in column I with the no of moles given in column II and mark the appropriate choice Column I Column II A 28 g of He 2 moles B 46 g of Na 7 moles C 60 g of Ca 1 mole D 27 g of Al 1 5 moles a A iv B iii C ii D i b A i B iii C ii D iv c A iii B ii C i D iv d A ii B i C iv D iii How many number of aluminium ions are present i ii iii iv

Physical Chemistry
Gaseous and liquid statesincorrect 0 00 points out of 10 00 Flag question Consider the following reaction equation 3 H g N g 2 NH3 g K 2 96 10 at a certain temperature At equilibrium the partial pressure of the hydrogen gas is 0 779 atm and the partial pressure of the nitrogen gas is 0 665 atm What is the partial pressure of ammonia in the equilibrium mixture at this temperature Select one 0 00965 atm 9 310 6 atm 0 00392 atm x 0 00346 atm 0 00305 atm

Physical Chemistry
Energetics3 Assertion A reaction which is spontaneous and by decrease of randomness must be accompanied exothermic Reason All exothermic reactions are accompanied by decrease of randomness

Physical Chemistry
Gaseous and liquid statesQuestion No 41 The ratio of most probable speed of Helium at 100 K to that of O2 at 400 K is O 2 1 O 3 1 O 4 1 O 2 1