Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Generalc A iii B ii C i D iv d A ii B i C iv D iii How many number of aluminium ions are present in 0 051 g of aluminium oxide a 6 023 x 100 ions c 6 023 x 10 ions b 3 ions d 9 ions

Physical Chemistry
EnergeticsThe molar enthalpies of combustion of C H g C graphite and H g are 1300 394 and 286 kJ mol respectively The standard enthalpy of formation of C H g would be Options 226 kJ mol 1 626 kJ mol 1 226 kJ mol 1

Physical Chemistry
General3 The anomeric carbon in D glucose is 1 C 1 carbon 2 C 2 carbon Correct sequence for reactivity of acid derivative is I RCO O II RCOCI 2 1 II II IV 1 II 1 II IV pH of a 10 10 M NaOH is nearest to 3 C 5 carbon III RCOOR 3 HI I IV III 4 C 6 carbon IV RCONH 4 I III II IV

Physical Chemistry
Generalthe acid 18 A 45 C 40 B 53 D 63 18 If 1 2 g of a metal displaces 1 12 L of hydrogen at NTP equivalent mass of the metal would be A 1 2 x 11 2 C 24 B 12 D 1 2 11 2 19 The atomic weight of a metal M is 27 and its equivalent weight is 9 the formula of its chloride will be
Physical Chemistry
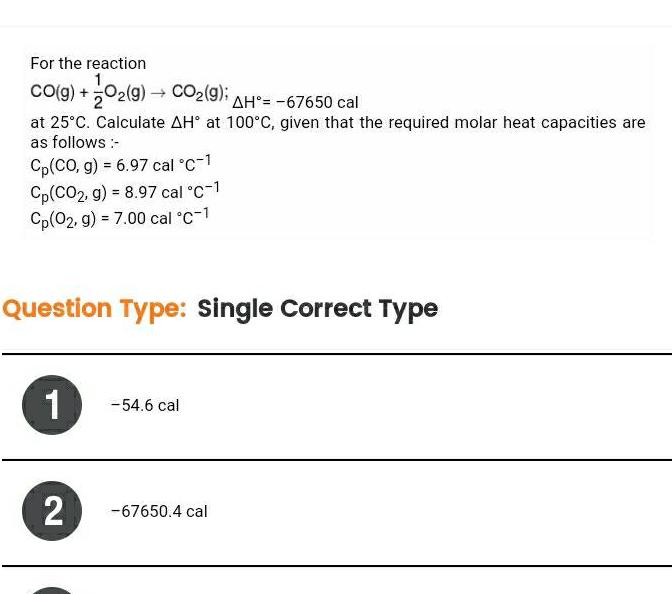
EnergeticsFor the reaction 1 CO g O2 g CO 9 AH 67650 cal at 25 C Calculate AH at 100 C given that the required molar heat capacities are as follows Cp CO g 6 97 cal C 1 Cp CO2 g 8 97 cal C 1 Cp 02 g 7 00 cal C 1 Question Type Single Correct Type 1 2 54 6 cal 67650 4 cal

Physical Chemistry
EnergeticsThe bond enthalpies of H H Cl Cl and H Cl are 435 243 and 431 kJ mol respectively The enthalpy of formation of HCl g will be Options 92 kJ mol 92 kl mol 1 247 kJ mol 1

Physical Chemistry
Equilibriumc 9 12 For the reaction AB g A g B g AB is 33 dissociated at a total pressure P Therefore P is related to K by which of following options P b P 3K d P 8K a P K c P 4K P d 1 6 P 3 2HI g IT P 1 3

Physical Chemistry
Chemical BondingC C CH C C CH OH 3 4 OH Which of the following chemical equation represents the formation of colloidal solution 1 Cu CuCl CuCl 2 2Mg CO 2MgO C 3 2HNO 3H S 3S 4H O 2NO 4 Both 2 3

Physical Chemistry
Equilibrium22 The standard Gibbs energy change at 300 K for the B C is 2494 2 J mol At a given time the composition of the reaction mixture is reaction 2A A M B 2M C M The reaction 2 2 proceeds in the R 8 34 J k mol e 2 718 a Forward direction because Q K b Reverse direction because Q K c Forward direction because Q K d Reverse direction because K

Physical Chemistry
GeneralT 7 moles of a tetra atomic non linear gas A at 10 atm and TK are mixed with 6 moles of another gas B at K and 5 atm in a closed rigid vessel witho 3 5T energy transfer with surroundings If final temperature of mixture was K then gas B is Assuming all modes of energy are active 6 monoatomic diatomic triatomic tetra atomic

Physical Chemistry
EnergeticsQuestion No 42 If the temperature of the gas molecules is increased then which is an incorrect statement about Maxwell Boltzmann distribution curve O The most probable speed increases O The fraction of molecules having higher speed decreases O The fraction of molecules having most probable speed decreases The entire curve shifts towards right

Physical Chemistry
Chemical kinetics16 Solubility curve of a hydrated salt in water with temperature is given The curve indicates that the solution process is Solubility g L 60 C Temperature a Exothermic b Endothermic c Endothermic till 60 C and endothermic after 60 C d Endothermic till 60 C and exothermic thereafter 17 Which of the following is not an endothermic reaction 21 An i to 1 1 x a C 27 22 Cha the a C 23 16 rev do a C

Physical Chemistry
General74 75 In the given reaction what is B HS OH 1 H C S TsCl Pyridine OTS 2 A OH B OH 3 4 The unit cell cube length for LICI NaCl type structure is 5 14 A Assuming anion cation contact calculate the ionic radius for chloride ion

Physical Chemistry
GeneralAt 300 K and 1 atm 15 mL of a gaseous requires 375 mL air containing 20 O by volume for complete combustion After combustion the gases occupy 330 mL Assuming that the water formed is in liquid form and the volumes were measured at the same temperature and pressure the formula of the hydrocarbon is CI CIT Main 2016 d CU

Physical Chemistry
Energetics30 For a certain reaction the change in enthalpy and change in entropy are 40 63 kJ mol and 100 JK What is the value of AG at 27 C and indicate whether the reaction is spontaneous or not a 10630 J mol spontaneous b 10630 J mol non spontaneous c 7990 J mol spontaneous d 7900 J mol spontaneous

Physical Chemistry
Energeticsd Constant temperature pressure and composition 6 The heat of combustion of solid benzoic acid at constant volume is 321 30 kJ at 27 C The heat of combustion at constant pressure is R a 321 30 300 b 321 30 300 R c 321 30 150 R d 321 30 900 R

Physical Chemistry
General1 The flux associated with a coil changes from 1 35 Wb to 0 79 Wb within s Then the charge 10 which flows in the coil if resistance of coil is 702 is 2 0 8 C 1 0 08 C 3 0 008 C 4 8 C A vessel contains 1g of oxygen at a pressure of 10 atm and a temperature of 47 C It is found that because of a leak the pressure drops to of its original value and the temperature falls to 27 C

Physical Chemistry
GeneralYou have a certain mass of helium gas in a rigid steel container You add the same mass of neon gas to this container Assuming that he temperature remains constant which of the following best describes what happens The volume in the container doubles The pressure in the container increases but does not double The pressure in the container drops The pressure in the container doubles

Physical Chemistry
EnergeticsC 33 4 Calculate the enthalpy change in kcal for the reaction XeFXe F F F The average Xe F bond enthalpy is 34 kCal mol first I E of Xe is 279 kCal mol electron gain enthalpy of F is 85 kCal mol and the bond dissociation enthalpy of F is 38 kcal mol a 292 kCal mol b 383 kCal mol c 521 kCal mol d 528 kCal mol

Physical Chemistry
Energetics3 Aerial 01 2 ethyl anthraquinol 4 Electrolysis Hay high current density One mole of a non ideal gas undergoes a change of state 2 0 atom 3 0L 95 K 4atm 5L 245K with a change internal energy AE 30 0L atm The change in enthalpy AH of the process in L atom is 1 40 0 3 44 0 1 CH MgBr 3 HLA 2 42 3 4 not defined because pressure is not constant Product

Physical Chemistry
Solid stateIf atom A is ideally fit in an octahedral void formed by atom B which crystallizes in fcc structure of radius 1 42 then what will be the minimum distance between centers of A and B in Use 2 1 41

Physical Chemistry
Gaseous and liquid states1 Assertion The equation PV nRT is not applicable to real gas Reason For real gases the attractive forces between the molecules cannot be neglected 8

Physical Chemistry
Generalc Ke 5 During complete combustion of one mole of butane 2658 kJ of heat is released The thermo chemical reaction for above change is a 2C H g 130 g 8CO g 10H O 1 AH 2658 0 kJ mol 13 b C H g 0 g 4CO g 5H O 1 A H 10 1329 0 kJ mol 13 c C H g O g 4CO g SH O 1 AH 10 2658 0 kJ mol d C H g O g 4CO g 5H O l AH 2658 01 sell

Physical Chemistry
Gaseous and liquid statespartial pressures 14 Assertion 1 4th of the initial mole of the air is expelled if air present in an open vessel is heated from 27 C to 127 C Reason Rate of diffusion of a gas is inversely proportional to the square root of its molecular mass

Physical Chemistry
Generalc 6 94 kg d 16 8 kg A balanced equation for combustion of methane is given below CH 20CO1H O Which of the following statements is not correct or the basis of the above chemical equation a b c d One mole of CH reacts with 2 moles of oxygen to give one mole of CO and 2 moles of water One molecule of CH reacts with 2 molecule of oxygen to give one molecule of CO and 2 molecules of water 22 41 of methane reacts with 44 8 L of oxyge to give 44 8 L of CO and 22 4 L of water 16 g of methane reacts with 64 g of O to giv 44 g of CO and 36 g of water

Physical Chemistry
Equilibriumhe degree of hydrolysis of salt of weak acid and weak base in its 0 1M solution is found to be 50 If the molarity of th olution of same salt is 0 5M then the percentage degree of hydrolysis of the salt should be 1 2 3 100 50 25

Physical Chemistry
GeneralAt 1000 K water vapour at 1 atm has been found to be dissociated into H and O2 to the extent of 3 x 10 6 Calculate the free energy decrease of the system assuming ideal behaviour 1 AG 90 060 cal 2 AG 20 cal 3 AG 480 cal 4 AG 45760 cal

Physical Chemistry
GeneralThe rate constant the activation energy and the frequency factor of a chemical reaction at 25 C are 3 0 x 10 4 s 104 4 KJ mol and 6 0 x 10 4 s1 respectively The value of the rate constant as T 1 8 is A B C D 2 0 x 1018 S 1 x 10 4 S 1 6 0 x 1 infinite 3 6 x 1030 5 1 X

Physical Chemistry
Gaseous and liquid statesA vessel has 6 g of oxygen at a pressure P and temperature 400 K A small hole is made in it so that O leaks out How much O leaks out if the pressure is and temperature is 300 K P 2 1 5 g 3 2 g 2 4 g 4 3 g

Physical Chemistry
Energetics34 The volume of a gas decreases from 500 cc to 300 cc when a sample of gas is compressed by an average pressure of 0 6 atm During this process 10 J of heat is liberated The change in internal energy is a 2 16 J b 12 156 J c 2 16 J d 101 3 J

Physical Chemistry
SolutionsA 0 001 molal aqueous solution of a complex M4 has the freezing point of 0 0054 C If th primary valency of the salt undergoes 100 ionization and K for water 1 8 Kmolal the corre representation of complex is 1 M 3 MA JA 4 MA 4 2 MA 4 Copper pyrite ore is concentrated by

Physical Chemistry
GeneralIf bond energies of C C and C H bonds are 336 2 kJ and 416 2 kJ respectively then the value of heat of atomisation of ethane is 1 752 4 kJ mol 2 2161 kJ mol 3 2001 kJ mol

Physical Chemistry
Gaseous and liquid statesThe cylinder contains 100gm of an ideal gas mol wt 40 gm mol at 27 C and 2atm pressure In transportation the cylinder fell and a dent was created The valve present cannot keep the pressure greater than 2atm Hence 10 gm of a gas got leaked out The volume of the container before and after dent is 4 27 7L 27 7L 1 30 8L 27 7L 2 2771 3081 3 30 ST 3081 Which of the following constitutes a set of amphoteric species

Physical Chemistry
Gaseous and liquid statesOne mole of an ideal monoatomic gas expands isothermally against constant external pressure of 1 atm from initial volume of 1L to a state where its final pressure becomes equal to external pressure If initial temperature of gas is 300 K then total entropy change of system in the above process is R 0 082 L atm mol 1 K 1 8 3 J mo1 K 1 1 0 2 Rin 24 6 3 Rin 2490 4 RIn 24 6

Physical Chemistry
Solid stateConsider a corner atom of Ist layer of an HCP unit cell showing alternate AA layers Find i Find identical atoms III layer with respect to the distances from the atom 1 ii Arrange the distances in ascending order f a og slo Atom 1 O d b Layer A Layer A

Physical Chemistry
GeneralSome Basic Concepts of Chemistry gram afom 61 How many grams of CaO are required to react with 852 g of P 0 0 a 852 g b 1008 g c 85 g d 7095 g 68 The cal a

Physical Chemistry
GeneralSteam distillation was carried out for an immiscible liquid P and it was observed that 100 ml of the distillate contained 28 ml of P The boiling point o the distillation was found to be 95 C at a pressure of 750 torr At 95 C assum that the vapor pressure exerted by water is 700 torr If the density of liquid P is 2 5 g ml then select the correct statements Vapor pressure of pure P at 95 C is 50 torr Molar mass of liquid P is 200 g mol Molar mass of the liquid P is 280 g mol Mass percent of P in the distillate is 49 3 approx

Physical Chemistry
Equilibriumplain which combination is the best choice to prep a buffer with a pH of 9 0 a NH3 NH4Cl pKb for NH3 4 75 b C5H5N C5H5NHCI pKb for C5H5N 8 76 c HNO2 NaNO2 pKa for HNO2 3 33 d HCHO2 NaCHO2 pKa for HCHO2 3 74

Physical Chemistry
Atomic StructureIn the Bohr s model for unielectronic species following symbols are used Radius of nth orbit with atomic number z Potential energy of electron in nth orbit with atomic number z Kinetic energy of electron in nth orbit with atomic number z Velocity of electron in nth orbit with atomic number z Time period of revolution of electron in nth orbit with atomic number z Calculate z in all in cases 8 1 Pn z Unz n z V n z T n z K1 2 1 U 2 K z iii V V 1 9 1 V3 ii 2 12 1 1 8 iv T 2 T z 9 32 T2

Physical Chemistry
GeneralThermal decomposition of dibrom coinic acid DBSA taking place according to the following equation obeys first order kinetics CH Br COOH a c CH Br COOH CBrCOOH The progress of reaction may be followed by means of alkali titration of the solution definite volume of reaction mixture at various time intervals If To and T be the ml of alkali solution at zero time and at any time t respectively and a and a x be the concentrations of DBSA at zero time and at any time t respectively then the value of a is T 3T 2T CHCOOH To T T b HBr d T FR T T 2T T a X

Physical Chemistry
Atomic Structure54 55 An organic compound A contains 20 C 46 66 N and 6 66 H It gave NH gas on heating with NaOH The organic compound A could be 1 CH CONH 2 C H CONH 3 NH CONH 4 CH NHCONH If the temperature of an ideal gas in a sealed rigid container is increased to 1 5 times the initial value

Physical Chemistry
Surface chemistry64 Which of the following graph represents the variation of amount of chemisorption of a gas by a solid with temperature under constant pressure 3 x m IS xm

Physical Chemistry
EnergeticsWhich of the following statements is are incorrect Options Absolute value of enthalpy cannot be determined Absolute value of internal energy cannot be determined Absolute value of entropy can be determined Internal energy enthalpy and

Physical Chemistry
Energeticsc 676 5 kJ d 676 5 kJ 54 When 50 cm of 0 2 N H SO is mixed with 50 cm of IN KOH the heat liberated is a 11 46 kJ b 57 3 kJ c 573 J d 53 J 55 The correct relationali

Physical Chemistry
General3 262 5 4 41 2 1 524 1 2 41 2 Which of the following is not an actinoid 1 Curium Z 96 3 Uranium Z 92 A chloride dissolves appreciable in cold water When placed on platinum wire in Bunsen flam distinctive colour is noticed Then the cation is 1 Mg 2 Ba 3 Ag 4 Ca In the chemical reaction 2 Californium Z 98 4 Terbium Z 65

Physical Chemistry
General16 If both oxygen and helium gases are at the same temperature the rate of diffusion of O is very close to a 4 times that of He b 2 times that of He c 0 35 times that of He d 8 times that of He

Physical Chemistry
Surface chemistryr is is of gy 41 A gas is collected by downward displacement of water Select the correct expression for p according to figure d 13 6 g cm Hg b Pas Gas H O d 1 g cm h a Pgas Patm Aqueous tension 13 6 Palm hdg cm C Pgas Patm Aqueous tension X d None of these h 13 6

Physical Chemistry
SolutionsEXAMPLE 2 30 Phenol associates in water to double molecules The values of observed and calculated molecular weight of phenol are 161 84 and 94 respectively The degree of association are 161 84 and 94 respectively The degree of association of phenol will be a 60 b 84 c 45 d 80

Physical Chemistry
Generalare prepared using MgSO4 and MgSO4 7H O with water In first case 91 kJ heat evolved while in 2nd case 13 kJ heat is absorbed enthalpy of hydration of MgSO4 will be Options 78 kJ mole 1 78 kJ mole 1 104 kJ mole 1
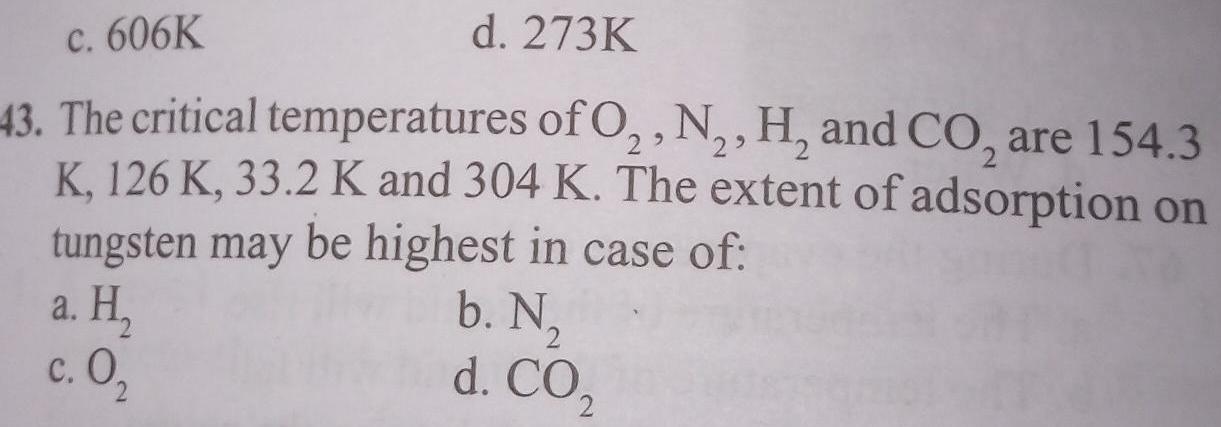
Physical Chemistry
Generalc 606K d 273K 43 The critical temperatures of O N H and CO are 154 3 K 126 K 33 2 K and 304 K The extent of adsorption on tungsten may be highest in case of b N d CO a H c 0