Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Gaseous and liquid statesd N O CO C3H8 36 The root mean square speed of an unknown gas at 27 C is x cm s The temperature at which its rms speed will be 3x cm s is a 2700 K c 2700 C 7 For H O b 270 K d 900 C

Physical Chemistry
Generalb 6 023x10 15 x 10 2 c 7 5 x 102 15 x 10 2 d 15 x 1022 7 5 x 10 50 Total number of atoms present in 34 g of NH is a 4x10 b 48x10 c 2 x 10 d 48 x 1023 51 What will be the mass of 100 atoms of hydrogen

Physical Chemistry
EquilibriumWhat is the concentration of Ba CN 2 in the aqueous solution of the mixture of Ba CN 2 and 0 1 M HCN having pH 5 Given pkb for CN at 25 C is 8 2 3 4 10 2 M 102 M 2x 10 2 M 5 x 10 3 M

Physical Chemistry
GeneralWhich of the following statements is invalid 1 The more stable the carbocation the faster it is formed 2 Propyl cation changes to more stable isopropyl carbocation by 1 2 shift of a hydrogen 3 Isopropyl chloride reacts with sodium ethoxide to form 1 ethoxypropane 4 Propyl halides reacts with sodium ethoxide to form 1 ethoxypropane

Physical Chemistry
General100 ml of an ideal gas is heated in an open vesse from 300 K to 400 K The volume of gas that wil remain in the vessel is 1 133 ml 3 33 ml 2 100 ml aldellou 4 67 ml

Physical Chemistry
EnergeticsThe following information is given for antimony at 1 atm AHvap 1440 00 C 1 605 10 J g T 1440 00 C Tm 631 00 C Specific heat solid 0 2090 J g C Specific heat liquid 0 2590 J g C AHfus 631 00 C 161 1 J g A 34 20 g sample of solid antimony is initially at 611 00 C If 5 179 x 10 J of heat are added to the sample at constant pressure P 1 atm which of the following is are true Select all that apply The sample is a liquid The sample is at a temperature greater than 631 00 C The sample is a solid The sample is a solid in equilibrium with liquid The sample is at a temperature of 631 00 C

Physical Chemistry
Generalkcal mole and 310 0 kcal mole 1 respectively then Options Ethane is better welding gas having more magnitude of AcHo Ethyne is better welding gas having more value of AcH Ethyne is better welding gas having more magnitude of AcH0 unit weight Fthane is better welding gas as here

Physical Chemistry
Solid stateScandium oxide Sc O3 crystallises with the oxide ions in a closed packed array with the scandium ions in octahedral holes What fraction of the octahedral holes are filled 1 All 2 2 3 1

Physical Chemistry
Generald A iv B iii 46 The number of oxygen atoms present in 1 mole of oxalic acid dihydrate is a 6x102 c 7 22 x 10 b 6 022 x 10 d 36 13 x 10 3

Physical Chemistry
Generald atomic masses cannot be weighed accurately 39 Oxygen occurs in nature as a mixture of isotopes OO and O having atomic masses of 15 995 u 16 999 u and 17 999 u and relative abundance of 99 763 0 037 and 0 200 respectively What is the average atomic mass of oxygen a 15 999 u b c 17 999 u d 16 999 u 18 999 u

Physical Chemistry
General57 What will be the weight of CO having the same number of oxygen atoms as present in 22 g of CO a 28 g c 44 g b 22 g d 72 g 58 Match the mass of elements given in column I with

Physical Chemistry
Atomic Structured 4 0 51 The most probable velocity of a gas molecule at 298 Ki 300 m sec Its RMS velocity in m s is a 420 b 245 c 402 d 367 52 The ratio of

Physical Chemistry
General0 02N A A 59 Which is mismatched regarding the position of the element as given below a X Z 89 f block 6th period b Y Z 100 f block 7th period c Z Z 115 d block 7th period d Both a c 64 W alu a A C F 65 Ger peri not a N c N
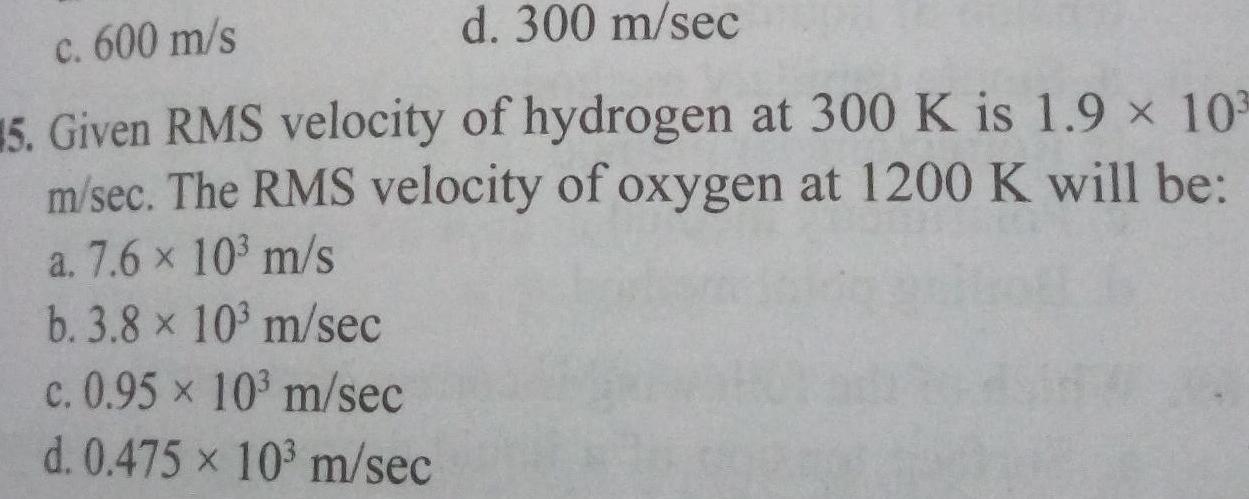
Physical Chemistry
Gaseous and liquid statesd 300 m sec c 600 m s 15 Given RMS velocity of hydrogen at 300 K is 1 9 103 m sec The RMS velocity of oxygen at 1200 K will be a 7 6 10 m s b 3 8 x 10 m sec c 0 95 x 10 m sec d 0 475 x 10 m sec

Physical Chemistry
GeneralQue 15 If the nitrogen atom had electronic configuration 1s7 it would have energy lower than that of the normal ground state configuration 1s2 2s2 2p3 because the electrons would be closer to the nucleus yet 1s7 is not observed because it violates Heisenberg uncertainty principle Hund s rule Pauli exclusion principle

Physical Chemistry
Gaseous and liquid states5 0 30 g of gas was found to occupy a volume of 82 0 ml at 27 C and 3 atm pressure The molecular mass of a gas is a 60 c 90 b 30 d Unpredictable

Physical Chemistry
Gaseous and liquid states14 2 g of hydrogen diffuse from a container in 10 min How many gram of oxygen would diffuse through the same container in the same time under similar conditions a 0 5 g c 6 g b 4 g d 8 g

Physical Chemistry
Generala 375 K c 546 K b 750 K d 408 K 36 If the density of a gas A is 1 5 times that of B then molecular mass of A is M The molecular mass of B will be a 1 5M b M 15 d M 3 c 3M

Physical Chemistry
General6 In a mixture of gases the volume content of a gas is 0 06 at STP Calculate the number of molecules of the gas in 1 L of the mixture a 1 613 x 102 c 1 61 x 1027 b d 6 023 x 1023 1 61 x 10 9

Physical Chemistry
GeneralRearrange the following I to IV in the order of increasing masses I 0 5 mole of 03 II 0 5 gm atom of oxygen III 3 011 x 1023 molecules of O IV 5 6 litre of CO at STP A II IV III I C IV II III I B II I IV III D I II III IV

Physical Chemistry
General3 The diagram shows the thermometer before and after adding the ammonium chloride in C C C 10 10 20 20 Fig 4 40 40 M i Record each of the temperatures and determine the fall in temperature Temperature before adding the ammonium chloride Temperature after adding the ammonium chloride Fall in temperature Covert the fall in temperature it to Fahrenheit m 50

Physical Chemistry
Gaseous and liquid statesc 67 2 L d 33 6 L 4 The volume of a gas at 0 C is 273 mL The volume of the gas at 27 C and at same pressure would be a 573 mL b 300 mL c 546 mL d 327 mL

Physical Chemistry
GeneralFig 1 i State three processes by which the dish and its contents could lose heat to the surroundings 2 Here are some statements about energy Select words from the following list and complete the statements I chemical electrical geothermal heat hydroelectric light movement kinetic position potential strain tidal wave a A coal fire converts energy and b When a ball falls from rest its energy into energy energy increases and its energy decreases c The source of energy in which hot rocks under the Earth s surface heat water to produce

Physical Chemistry
General4 Iron can be obtained by reduction of iron oxide Fe O with CO according to the reaction FeO 400 3Fe 4CO How many kg of Fe 0 should be heated with CO to get 3 kg of tron a 8 12 kg c 6 94 kg b 4 14 kg d 16 8 kg
Physical Chemistry
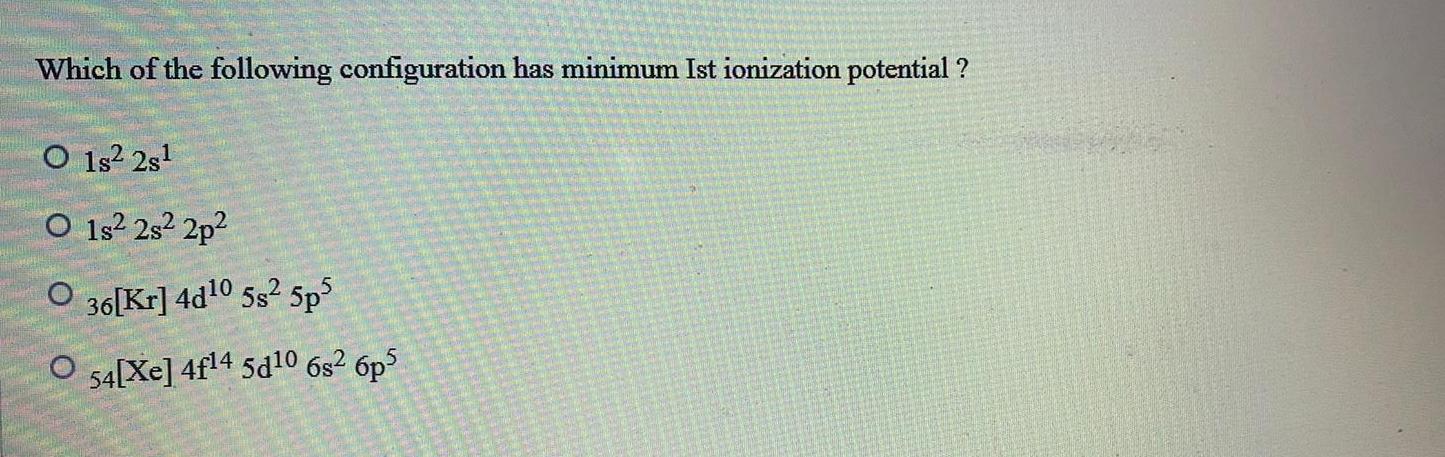
Atomic StructureWhich of the following configuration has minimum Ist ionization potential O1s 2s O1s 2s 2p O 36 Kr 4d 0 5s 5p5 O 54 Xe 4f14 5d10 6s 6p5

Physical Chemistry
General54 1 4 moles of phosphorus trichloride are present in a sample How many atoms are there in the sample a 5 6 c 2 4 x 1023 b 34 d 3 372 x 1024 CH

Physical Chemistry
EnergeticsThe standard reaction enthalpy of the reaction Zn s H O g ZnO s H g is 223 kJ mol and the standard reaction Gibb s functions is 33 kJ mol at 1520 K Assuming that both AH and AS remain constant estimate the minimum temperature in Kelvin above which the equilibrium constant becomes greater than one

Physical Chemistry
General48 Which of the following gases will have least volume if 10 g of each gas is taken at same temperature and pressure a CO c CH b N d HG molec es and toms

Physical Chemistry
Generalc 19300 d 10 33 The ratio of masses of hydrogen and magnesium deposited by the same amount of electricity from H SO4 and MgSO4 in aqueous solution are a 1 8 b 1 12 c 1 16 d none of these The cell reaction for the given cell is spontaneous if

Physical Chemistry
Gaseous and liquid statesa 1 5M b M s c 3M d M 3 37 The approximate temperature at which 1 mol L of a sample of pure ideal gas exhibits a pressure of 101 325 K Pa is a 12 2 K b 122 K c 244 K d 300 K

Physical Chemistry
SolutionsThe vapour pressure of benzene and toluene in pure state are 700 and 600 mm of Hg respectively When mol of benzene and 4 mol of toluene are mixed together then the vapour pressure of solution will be 680 mm 650 mm 620 mm 600 mm

Physical Chemistry
Solid stateThe density and edge length values for a crystalline element with fcc lattice are 10 g cm and 400 pm respectively The number of unit cells in 32 g of this crystal is A 8 1023 B 5 x 1022 C 8 1022 D 5 tune of defect observed in stoichiometric compounds is Schottky defect Schottky defect occur mainly in ionic

Physical Chemistry
GeneralOne gram of silver gets distributed between 10 cm of molten zinc and 100 cm of molten lead at 800 C The percentage of silver in the zinc layer is approximately Given Partition coefficient of Ag in Zn and Pb is 300 A 89 B 91 C 97 D 94 Owl of athul oploohol to formethyl acetate The density of acid and alcohol are

Physical Chemistry
GeneralSir in Cr NH3 6 how number of unpaired electron is 3 As Cr have 3d configuration but due to strong ligand it should be paired and number of unpaired electron should be 1 T

Physical Chemistry
EnergeticsThe diamonds high pressure are formed from graphite under very Given that the densities of graphite and diamond are respectively 2 4 and 3 6 g cm and are independent of pressure AGO values for graphite and diamond are zero and 3 0 kJ mol respectively If the equilibrium pressure at which graphite is converted into diamond at 25 C is P bar then the value of 0 01P is

Physical Chemistry
GeneralThe vapour pressure of water at 293 K is 17 51 mm The lowering of vapour pressure of sugar is 0 0614 mm Calculate a The relative lowering of vapour pressure b The vapour pressure of the solution C The mole fraction of water

Physical Chemistry
Equilibrium3 Eight mole of chlorine Cl undergoes a loss and gain of 14 mole of electrons to form two oxidation states of chlorine CI Write down the two half reactions and equation for disproportionate of chlorine Cl

Physical Chemistry
General4 The owner of a small factory suggests installing a wind turbine to generate some of the electricity needed by the factory Discuss three of the factors that the owner will need to consider when deciding whether to install a wind turbine

Physical Chemistry
EnergeticsOne mole of a monatomic gas at pressure 2 atm 279 K is taken to final pressure 4 atm by a reversible path described by P V constant Calculate the magnitude of process AE W for the

Physical Chemistry
Gaseous and liquid states23 A constant pressure air thermometer gave a reading of 47 5 units of volume when immersed in ice cold water and 67 units in a boiling liquid The boiling point of the liquid will be 2 125 C 4 190 C 1 135 C 3 112 C

Physical Chemistry
SolutionsIntermolecular forces between n hexane and n heptane are nearly same as between hexane and heptane individually When these two are mixed which of the following is now true about the solution formed a It obeys Raoult s law i e p xp and p X P s b AH is zero c AV is zero fish for

Physical Chemistry
Chemical kineticsB is an reactant invovled in an reaction It is known that the reaction rate can be calculated by Rate dCB dt kCBb where CB is the concentration of B k is the apparent rate constant and b is the order of the reaction with respect to B When b 1 CB was measured as 8 7 3 31 and 1 45 mg L at 3 49 4 64 and 9 22 hours after the start of the reaction Please calculate the value of the apparent

Physical Chemistry
SolutionsThe vapour pressure of a solvent A is 0 80 atm When a non volatile substance B is added to this solvent its vapour pressure drops to 0 6 atm What is mole fraction of B in solution a 0 25 c 0 75 MP PMT 2000 01 UPSEAT 2003 MP PET 2003 J K CET 2010 Odisha JEE 2010 b 0 50 d 0 90

Physical Chemistry
Gaseous and liquid states2 1 Which of the following expression is true regarding g laws W weight M molar mass a C T M W T M W T M W T M W M W T M W 2 b T d T M W T

Physical Chemistry
SolutionsIntermolecular forces between n hexane and n heptane ares nearly same as between hexane and heptane individually When these two are mixed which of the following is not true about the solution formed a It obeys Raoult s law i e p xp and p X P b AH is zero c AV is zero d It forms minim

Physical Chemistry
Gaseous and liquid statesWhen chromium metal is added to nitric acid the following reaction takes place Cr s 6 HNO aq Cr NO3 3 aq 3 H O 1 3 NO g Calculate the volume of NO gas in L collected over water at 40 0 C when 48 7 g of chromium is added to excess nitric acid if the total pressure is 675 0 torr The vapor pressure of water at 40 0 C is 55 3 torr 1 st 4 7

Physical Chemistry
Energetics5 a The diagram to the right represents a diver s motion from the top of a high diving board into a pool of water At what point does the diver have the greatest kinetic energy and the least potential energy

Physical Chemistry
Generalc 22 4 L d 4 L 5 If V is the volume of a given mass of gas at 273 K at a constant pressure then according to Charle s law the volume at 10 C will be a 11V b V 10 273 c V 10 273 d 283 273 Vo

Physical Chemistry
Chemical kineticsIron 59 is a radioisotope that is used to evaluate bone marrow function The half life of iron 59 is 44 5 days If you begin with 35 8 mg of this isotope what mass remains after 87 2 days have passed

Physical Chemistry
Atomic Structure40 The ratio of de broglie wavelength of a deuterium atom to that of a particle when the velocity of former is five times greater than that of latter is a 4 b 10 2 c 2 d 0 4