Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Electrochemistry3 In an electrolysis of acidulated water 4 48 L of hydrogen at STP was produced by passing a current of 2 14 A For how many hours was the current passed 1 4 3 6 2 3 4 5

Physical Chemistry
Solutionswwwing Determine the process occurring at each labeled point on this graph TEMPERATURE 2 3 COOLING TIME 4 5 A 01 24 03 Time Remaining Epo0000000 9 Gas neating Solid cooling Liquid heating Melting Vaporization Liquid cooling Condensation Freezing Gas cooling 2 points What process is occurring at point 5 Melting Gas cooling Solid cooling Freezing Solid heating Gas heating Vaporization 00000000 Liquid cooling

Physical Chemistry
GeneralType your answer Cooling curve Determine the process occurring at each labeled point on this graph TEMPERATURE 2 3 COOLING TIME 4 5 7 4 points What process is occurring at point 3 Vaporization 000000003 Gas heating Solid heating Liquid heating Condensation Melting Freezing Gas cooling Solid cooling

Physical Chemistry
SolutionsPressure Phase diagram Determine the phase of matter or phases occurring at each labeled point on this phase diagram C A E B Temperature F T G D 17 4 points What phase or phases of matter is point A Gas and liquid Solid Solid and liquid Gas Gas and solid Liquid 00000000 Supercritical fluid Gas solid and liquid 18 4 points What phase or phases of matter is point B Solid liquid and gas Supercritical fluid 00000 Gas Gas and liquid Solid and liquid

Physical Chemistry
ElectrochemistryAt 25 C molar conductance of 0 1 molar solution of ammonium hydroxide aqueous is 9 54 ohm cm mol and at infinite dilution its molar conductance is 238 ohm cm mol The degree of ionisation of ammonium hydroxide at the same concentration and temperature is a 4 008 b 40 800 c 2 080 d 20 800 NEET 2013

Physical Chemistry
General1 4h 2 3h 3 2h CHEMISTRY Setting of plaster of Paris is 1 Dehydration 3 Combination with atmospheric CO 4 2 2 Oxidation with atmospheric oxygen 4 Hydration to yield another hydrate

Physical Chemistry
General5 points Calculate the amount of energy in kJ needed to heat 510 9 g of gold Au from 25 0 C to 1 113 0 C The melting point of gold is 1 064 8 C SAu s 0 147 J g C SAu I 0 128 J g C AH fusion 13 5 kJ mol Do not write unit in answer Report your answer with 1 place past the decimal point Type your answer

Physical Chemistry
Chemical kineticsFor the reaction CH Br aq OH aq CH OH aq Br aq rate law is rate K CH3Br OH a How does reaction rate changes if OH is decreased by a factor of 5 b What is change in rate if concentrations of both reactants are doubled

Physical Chemistry
Solid stateIn a structure oxide ions are cubical closest packed whereas t th of tetrahedral voids are 1 8 1 occupied by A cation and of octahedral voids 2 are occupied by B 3 cations The formula for this compound is 1 ABO 4 2 AB 04 4 ABO

Physical Chemistry
Solid stateElement x y and z crystallize in primitive face centered and body centered unit cell respectively What would be the correct order of metallic radii if volume of each unit cell is same 1 y 3 r r r 2 ry r r 4

Physical Chemistry
Chemical kineticsCalculate the pH of buffer solution composed of 0 1M weak base BOH and 0 2M of its salt BA Kb 1 8 x 10 5 for weak base Define Pseudo first order reaction In a first order reaction 60 of reactant decomposes in 45 minutes Calculate the half life for the reaction

Physical Chemistry
Chemical BondingSchottky defect is shown by A strongly ionic compounds B C D compounds having high coordination number compounds containing cations and anions of almost similar size all of these Jhap

Physical Chemistry
Chemical BondingWhich of the following statements regarding Nitrogen pentoxide is not correct 1 Nitrogen pentoxide is a colourless deliquescent liquid 2 Nitrogen pentoxide is the anhydride of nitric acid 3 Solid N O is a covalent molecule 4 The molecule of N O in planar

Physical Chemistry
General0 25 g of an organic compound on heating with conc HNO3 and silver nitrate in carius tube gave 0 20 g of AgBr then the percentage of bromine in the compound is Atomic Mass of Ag 108 u Br 80 u 28 34 6

Physical Chemistry
Equilibriumd 3 0 10 7 is x and that in 0 1 M AgNO3 is y then which of the following is correct a x y b x y c x y d We cannot predict 145 What is the molarity of Fe CN in a saturated solution of Ag 4 Fe CN 6 Ksp 1 6 10 41 a 1 6 x 10 8 d 2 3 x 10 9 4 b 5 2 x 10 8 c 2 0 10 8 146 At 25 C Ksp for PbBr2 is equal to 8 x 10 5 If the salt is 80 din

Physical Chemistry
EquilibriumAn analytical chemist is titrating 189 8 mL of a 1 200M solution of hydrezoic acid HN with a 1 100M solution of KOH The pX of hydrazoic acid is 4 32 Calculate the pH of the acic solution after the chemist has added 89 21 mil of the KOH solution to it Note for advanced students you may assume the final volume equas the initial volume of the salution plus the volume of KOH solution added Round your answer to 2 decimal places 1 0 pH 5

Physical Chemistry
EnergeticsWhat is bond enthalpy Calculate C Cl bond enthalpy from following reaction CH4 g Cl2 g CH3Cl g HCl g AH 104 KJ mol if C H Cl Cl and H Cl bond enthalpies are 414 243 and 431 KJ mol respectively

Physical Chemistry
Chemical kineticsen pentoxide decomposes to O2 and O following first order kinetics N O g 2NO O g 0 2 mole of N Os was taken in 2 L vessel and heated at 200 K The concentration of N Os is measured at different intervals and the following graph was plotted 13 log N O Time Slope of straight line in graph A is 1 2 x 10 sec 1 what is half life of the reaction A 2 5 x 10 S B 2 5 x 10 3 S C 12 5 x 10 s D 2 5 x 104S

Physical Chemistry
Electrochemistry4 The molecule of N O in planar Two different electrolytic cells filled with molten Cu NO and molten AI NO respectively are connected in series When electricity is passed 2 7 g Al is deposited on electrode Calculate the weight of Cu deposited on cathode Cu 63 5 Al 27 0 g mol 1 190 5 g 2 9 525 g 3 63 5 g 4 31 75 g

Physical Chemistry
Solid state6 In cubic lattice atoms are arranged A at corners B at face centers C at edge and body centre and 2D at triads tetrahedral voids then the body diagonal touches 1 2A 2C 2D 3 2A B 2C 2 2A 2B C 4 2A C 2D in fc

Physical Chemistry
GeneralCooling curve Determine the process occurring at each labeled point on this graph TEMPERATURE 2 COOLING TIME 4 6 3 points What process is occurring at point 1 Freezing Liquid cooling 000000000 Vaporization Condensation Melting Liquid heating Solid heating Solid cooling Gas heating

Physical Chemistry
Equilibrium147 What is the molar solubility of Mn OH 2 Ksp 4 5 x 10 4 in a buffer solution containing equal amounts of NH4 and NH3 Kb 1 8 10 5 a 3 0 x 10 4 b 1 38 x 10 4 48 Find moles of NILL 3 c 1 38 x 10 d 7 3 x 10

Physical Chemistry
General140 The calculated spin only magnetic moment of Cr ion is 1 2 3 2 84 BM 3 87 BM 4 90 BM 5 92 BM 70 Brtne Hydro Hulep 1 Br 10 hydrolysis gives

Physical Chemistry
GeneralKMnO4 mol wt 158 oxidizes oxalic acid in acidic medium to CO and water as follows 5C 042 2MnO4 16H 10CO 2Mn 8H O What is the equivalent weight of KMnO4 A 158 B 31 6 C 39 5 D 79 vidion ono molo of ferrous oxalate FeG 0 completely in acidic

Physical Chemistry
General3 The figure shows a disc of radius 3R from which a circular hole of radius R is cut as shown in the figure The distance of the centre of mass of the remaining object from the point O is R R 6 A block A of mass 2m is hanging from a vertical massless spring of spring constant and is in 74 3R R Rm

Physical Chemistry
Equilibrium0 3 Then find out mole fraction of PCI 1 0 3 2 0 7 3 0 4 4 0 6 If 8 mol of PCl heated in a closed vessel of 10 L capacity and 25 of its dissociates into PCl3 and Cl at the equilibrium then value of Kp will be equal to 1 P 30 2 P 15 3 2 3P 4 3 2P In the reaction PCl5 PCl3 Cl the partial pressure of PCl3 Cl and PCI are 0 3 0 2 and 0 6 atm respectively at equilibrium If partial

Physical Chemistry
GeneralSelect the incorrect statement among the following Calcium plays important role in neuromuscular function Calcium concentration in plasma is regulated by calcitonin and parathyroid hormone All enzymes that utilise ATP in phosphate transfer require magnesium as the cofactor Sodium ions are the most abundant cations within cell fluids

Physical Chemistry
ElectrochemistryHgSO4 is 6 4 x 10 sp a 8 10 3 then d None of these b 6 4 x 10 5 c 8 x 10 6 130 The solubility of Ba 3 AsO 4 formula mass 690 is 6 9 x 10 2 g 100 mL What is the K 2 a 1 08 x 10 11 d 6 0 10 13 13 b 1 08 x 10 c 1 0 10 15 131 The solubility of AgBrO formula mass 236 is 0 0072 g in 1000 mL What is the K c 3 0 10 5 a 2 2 x 10 8 b 3 0 x 10 10 sp d 9 3 10 10

Physical Chemistry
GeneralThe IUPAC name of the following compound is CONH CHO 1 2 Carbamoylhexanal 3 2 Methy 6 oxohex 3 enamide 2 2 Carbamoylhex 3 en 4 6 keto 2 methyl hexa

Physical Chemistry
General33 A hydrocarbon contains 80 of carbon then the lukt hydrocarbon is 1 CHA 3 C H 6 2 C H 4 C H

Physical Chemistry
General1 In system A s 2B g 3C g at equilibrium if concentration of C is doubled then concentration of B at equilibrium 1 Double its original concentration 2 Half its original concentration 3 2 2 its original concentration 4 its original concentration 2 concer

Physical Chemistry
GeneralA solution contains 5 62 10 M ammonium sulfite and 7 70 10 M potassium carbonate Solid barium acetate is added slowly to this mixture What is the concentration of carbonate ion when sulfite ion begins to precipitate M carbonate

Physical Chemistry
General1 5 4 10 m 2 5 4 10 m 3 5 4 10 m 4 5 4 10 m If the dipole moment of Toluene and Nitro benzene are 0 43D and 3 93D respectively then what is the expected dipole moment of p Nitrotoluene 1 3 50 D 2 2 18 D 3 4 36 D 4 5 30 D Methanoic acid is heated with conc H SO to form 1 CO 2 CO 3 CH 4 COOH

Physical Chemistry
SolutionsMole fraction of benzene in the vapours ove r a solution of benzene and toluene is 0 4 If the original vapour pressure of benzene is 1 50mmHg and toluene is 50mmHg the mole fraction of benzene in the solution is a 0 4 b 0 75 c 0 25 d 0 18

Physical Chemistry
Solid stateequations is most applicable 1 AH AE 2 AH AS 3 AH AE The void space in a primitive unit cell is 1 48 void space 2 24 void space 3 96 void space In chelate therapy lead toxicity is removed by using the ligand Coo 4 Total W 0 4 50 void space

Physical Chemistry
EnergeticsThe difference between heats of reaction at constant pressure and constant volume of the following reaction would be 12CO2 g 6H O l at 25 C 2C6H6 1 150 g in kJ mol is a 7 43 c 5 72 b 4 72 d 8 43

Physical Chemistry
EquilibriumConsider the reaction below Which of the following would increase the partial pressure of B at equilibrium A s B g C g AH 0 A adding A B increasing the total volume of the container C increasing the temperature D removing C
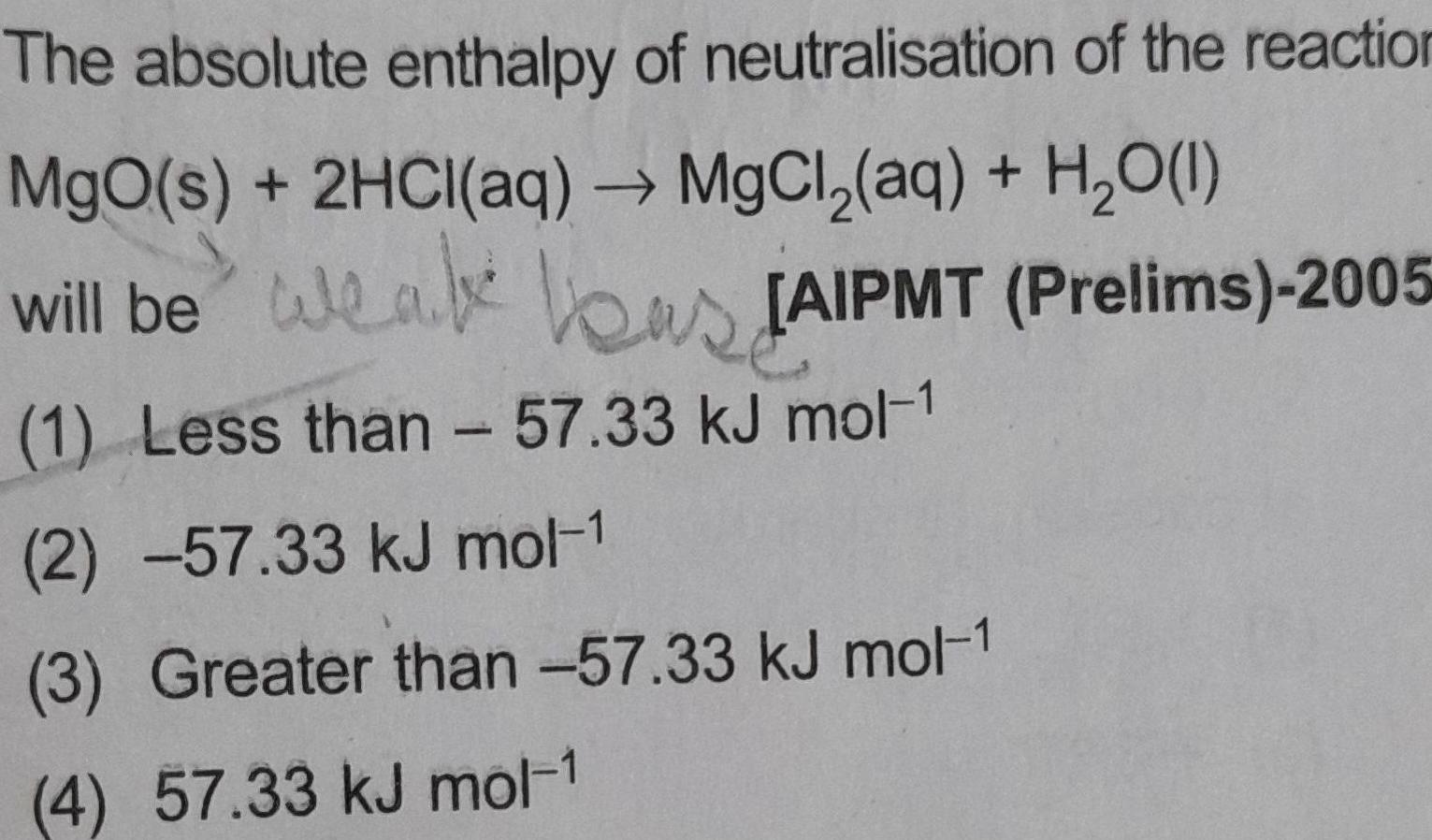
Physical Chemistry
Atomic StructureThe absolute enthalpy of neutralisation of the reaction MgO s 2HCl aq MgCl aq H O 1 will be weak as JAIPMT Prelims 2005 1 Less than 57 33 kJ mol 1 2 57 33 kJ mol 1 3 Greater than 57 33 kJ mol 1 4 57 33 kJ mol 1

Physical Chemistry
General10 0 mL of Na CO3 solution is titrated against 0 2 M HCl solution The following titre values were obtained in 5 readings 4 8 mL 4 9 mL 5 0 mL 5 0 mL and 5 0 mL Based on these readings and convention of titrimetric estimation the concentration of Na CO3 solution is mM Round off to the Nearest Integer n

Physical Chemistry
GeneralSolutions 64 g of methane g is mixed with 16 moles of oxygen g The mixture is then allowed to react to produce maximum amount of carbon dioxide g and water g Choose the correct statement regarding the above case Mole fraction of methane g in the initial reactant mixture is 0 25 Mass percent of oxygen in the limiting reagent is 100 Mass of carbon dioxide g produced is 88 g Mass percent of water a in

Physical Chemistry
Chemical BondingThe number of species below that have two lone pairs of electrons in their central atom is Round off to the Nearest Integer SF BF4 CIF ASF PC15 BrF5 XeF SF6 4 en 2

Physical Chemistry
Chemical kineticsven ver A reaction has a half life of 1 min The time required for 99 9 completion of the reaction is min Round off to the Nearest Integer Use In 2 0 69 In 10 2 3

Physical Chemistry
Energetics4 A mixture of phenol and Mg Me Br If AH for H O and H Oare 188 kJ mol and 286 kJ mol What will be the enthalpy change of the reaction 2H O 1 2H O 1 0 g 1 196 kJ 2 494 KJ 3 146 kJ 4 98 kJ

Physical Chemistry
Chemical Bonding1 7 Ethyl 2 4 5 6 tetramethyldeca 1 8 diene 2 4 Ethyl 5 6 7 9 tetramethyldeca 2 9 diene 3 2 4 5 6 tetramethyl 7 ethyldeca 1 7 diene 4 None of these Which of the following sulphates has the highest solubility 1 BESO 2 MgSO 3 BaSO 4

Physical Chemistry
GeneralThe Balmer series in the hydrogen spectrum corresponds to the transition from m 2 to n 3 4 This series lies in the visible region Calculate the wave number of line associated with the transition in Balmer series when the electron moves to n 4 orbit RH 109677 cm

Physical Chemistry
Solid stateSquare packed sheets are arranged one on the top of the other such that a sphere in next layers rests on the top of spheres in previous layer Identify the type of arrangement and find the coordination number 1 simple cubic 8 2 simple cubic 6 3 body centerede cubic 8 4 face centered cubic 12

Physical Chemistry
GeneralWhich of the following combinations of solutes and solvents could result in a solution Check all that apply H O and hexane CH CH CH CH CH CH Ethanol CH CH OH and H O H O and Na SO Hexane CH CH CH CH CH CH and ethane CH CH Do you know the answer

Physical Chemistry
Chemical Bonding0 The atomic radii of transition elements from Cr to Cu are almost equal because 1 Increased effective nuclear charge is balanced by decreased screening effect of electrons in n 1 d orbitals 2 Increased effective nuclear charge is balanced by increased screening effect of n 1 d orbitals 3 Decreased effective nuclear charge is balanced by increased screening effect of electrons in n 1 d orbitals 4 None of these Benzene diazonium chloride on boiling with dilute sulphuric acid gives

Physical Chemistry
Atomic Structure1 30 The measurement of the electron position is associated with an uncertainty in momentum which is equal to 1 x 10 18 g cm s 1 The uncertainty in electron velocity is Mass of an electron is 9 10 28 g AIPMT Prelims 2008 1 1 x 1011 cm s 2 1 x 10 cm s 3 1 x 106 cm s 4 1 x 105 cm s 1 Consider the following cots of quantu The is orb 1 2 3 4 Questi 35 U 10

Physical Chemistry
GeneralA certain reaction B is getting converted to B 4 in solution The rate constant of this reaction is measured by titrating a volume of the solution with reducing agent which reacts only with Bnt B 4 In this process it converts B to B n 2 and B n 4 to B n 1 At t 0 the volume of reagent consumed is 26 mL and at t 23 minutes volume used is 35mL Bn to B 4 is first order reaction The rate constant is 1 33x10 min log 1 3 0 1139 The value of x is