Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Atomic StructureWhat minimum tube voltage is required to excite the KB and LB series of lines for rubidium Rb A 15 0 kV for KB 1 75 kV for LB B 1 75 kV for KB 15 0 kV for LB C 15 0 kV for KB 15 0 kV for LB D 1 75 kV for KB 1 75 kV for LB

Physical Chemistry
SolutionsA gaseous hypothetical chemical 2A4B C is carried out in a closed vessel The concentration of B is found to be increase by 5 x 10 mol L in 10 seconds The rate of appearance of B is 5 x 10 4 mol L sec 5 x 10 5 mol L sec O 6 x 10 5 mol L sec 1 4 x 104 mol L sec 1 O O 1

Physical Chemistry
Atomic StructureTwo solids dissociate as follows A s B g C g Kp x atm D s C g E g K yatm P2 The total pressure when both the solids dissociate simultaneously is a x y atm c x y atm 2019 Main 12 Jan I b x y atm d 2 x y atm

Physical Chemistry
EnergeticsUse the collision theory of gas phase reactions to calculate the theoretical value of the second order rate constant for the reaction D2 g Br2 g 2 DBr g at 450 K assuming that it is elementary bimolecular Take the collision cross section as 0 30 nm the reduced mass as 3 930 u and the activation energy as 200 Kj mol Select one a 1 7 X 10 2 dm mol s1 b 4 7 X 1013 dm mol st c 4 6 X 1013 dm mor s1 d 6 4 X 10 2 dm mol s1 e 3 5 X 10 1 dm mol g

Physical Chemistry
Gaseous and liquid statesIn preparation for a demonstration your professor brings a 1 50 L bottle of sulfur dioxide into the lecture hall before class to allow the gas to reach room temperature If the pressure gauge reads 198 psi and the lecture hall is 20 C how many moles of sulfur dioxide are in the bottle In order to solve this problem you will first need to calculate the pressure of the gas Hint The gauge reads zero when 14 7 psi of gas remains mol

Physical Chemistry
GeneralEqual volumes of three monobasic acids of pH 3 4 5 are mixed in a vessel The pH of the resultant solution will be log 3 7 0 57 3 91 5 27 6 81 3 43

Physical Chemistry
Surface chemistrySpontaneous adsorption of gas on solid surface is an exothermic process because Question Type Single Correct Type 1 Enthalpy increases for system 2 Entropy increases for gas 3 Entropy decreases for gas

Physical Chemistry
General2 Al O3 is reduced by electrolysis at low potentials and high currents If 4 0 10 amperes of current is passed through molten Al O3 for 6 hours what mass of aluminium is produced Assume 100 current efficiency at mass of Al 27 g mol a 8 1 x 10 g c 1 3 x 104 g g b 2 4 x 105 g d 9 0 10 g 2009

Physical Chemistry
Equilibrium25 Equal volumes of 10 v v of HCl is mixed with 10 v v NaOH solution If density of pure NaOH D can t be predicted 1 5 times that of pure HCI then the resultant solution be A basic B neutral C acidic

Physical Chemistry
General7 The solubility product of AgCl is 10 10 M2 The minimum volume in m of water required dissolve 14 35 mg of AgCl is approximately a 0 01 b 0 1 c 100 d 10 What is the molar solubility of Fe OH 2 Ksp 8 0 x 10 16 at pH 13 0

Physical Chemistry
SolutionsM 9 If the degree of association is 70 for the reaction 2A the solute A is a 0 30 c 0 35 Sol d A 2 the van t Hoff factor for 7 May 2018 Shift 1 b 0 70 d 0 65 24 4 2 van t Hoff factor i 70 0 7 per unit i e a 0 70 Given where a degree of association n number of units undergo for association a 0 70 i 1 a 1 0 70 71 2 i 0 30 0 35 0 65

Physical Chemistry
GeneralA solution contains 1 30 10 2 M potassium sulfide and 1 12 10 2 M sodium hydroxide Solid manganese II acetate is added slowly to this mixture A What is the formula of the substance that precipitates first formula B What is the concentration of manganese II ion when this precipitation first begins Mn M

Physical Chemistry
Equilibriumindicate whether solutions with each of the following ion concentrations are neutral acidic or basic at 25 C Note that 1 means acidic 1 means basic and 0 means neutral a OH 1 68 10 10 M b H 7 94 x 10 10M c H 1 10 M d H 4 19 10 M e OH 9 73 10 22 M

Physical Chemistry
GeneralA complex is repesented as CoCl3 xNH3 Its 0 1 molal solution in water shows AT 0 558 If the cryscopic and ebullioscopic constants for H O are 1 86 K kg mor and 0 52 K kg mor respectively identify the correct versions assuming 100 ionisation of the complex Hexaaminecobalt III chloride is the IUPAC name for the complex The above solution will boil between 100 C to 101 C The central metal ion is d sp hybridised The coordination sphere is incapable of exhibiting geometrical isomerism

Physical Chemistry
GeneralIn alkaline medium CIO oxidises H O to O and itself gets reduced to Cl How many moles of H O are oxidised by 1 mole of CIO 2 1 5 1 1 3 25 4 5

Physical Chemistry
EquilibriumPotassium sorbate C6H7KO2 is an additive used to prevent molding of cheese Its conjugate acid monoprotic has Ka 1 74x10 5 Find the pH of the aqueous solution containing 2 55 g of potassium sorbate in 500 0 mL

Physical Chemistry
GeneralThe ratio of difference in energy of electron between first and second Bohr s orbit to that between second and third Bohr s orbit is A 1 3 B 27 5 skipped

Physical Chemistry
Atomic StructureFor 3s orbital of hydrogen atom the normalised wave function is given by 3 2 1 1 18r 2r 813 80 10 The above mentioned orbital 3s has two nodes at 2ao and xao Find the value of x 4 Use 3 3 5 W3s 27 C

Physical Chemistry
Surface chemistryU m the 32 If K and K are maximum kinetic energies of mitted photoelectrons emitted when lights of wavelength and respectively incident on a metallic surface 13 then a K K 3 c K 3K n the b K K 3 d K 3K

Physical Chemistry
EquilibriumIf the concentration of OH ions in the reaction Fe OH 3 s Fe aq 3OH aq is 1 4 concentration of Fe3 will increase by decreased by 1 4 times 3 16 times times then equilibrium AIPMT Prelims 2008 2 8 times 4 64 times

Physical Chemistry
EquilibriumCalculate the hydronium ion concentration and the pH at the equivalence point in a titration of 50 0 mL of 0 040 M NH with 0 04 MHCI K 1 8x10 Please don t paste a solution from inter net I already looked at them and they h ave incorrect solutions I solved it by finding concentration of N H4Cl at equivalnce point 2 10 3 and found the pH using hydrolysis formula f or SAWB and the pH came as 5 97 but t

Physical Chemistry
Generala 1 6 10 b 5 2 x 10 8 9 c 2 0 10 8 d 2 3 10 146 At 25 C Ksp for PbBr is equal to 8 10 5 If the salt is 80 dissociated what is the solubility of PbBr2 in mol litre 71 3 10 4 2 1 6 1 6 10 5 1 6 1 6 1 3 1 3 10 5 1 6 x 1 6 71 2 b c 10 4 0 8 0 8 d 147 What is the molar solubility of Mn OH Ksp 4 5 10 4 in a buffer solution containing

Physical Chemistry
EquilibriumThe ionization constant of ammonium hydroxide is 1 77 x 10 5 at 298 K Hydrolysis constant of ammonium chloride is AIPMT Prelims 2009 1 6 50 10 12 3 5 65 x 10 12 2 5 65 x 10 13 4 5 65 10 10

Physical Chemistry
Electrochemistrya 1 0 b 1 29 c 134 What is the molarity of Fions in a saturated solution of BaF Ksp 1 0 106 a 1 0 10 2 b 1 0 x 107 3 c 1 26 x 10 2 d 6 3 10 3 135 What is the molarity of F in a saturated solution of InF3 Ksp 7 9 10 0

Physical Chemistry
Equilibrium141 What is the molar solubility of Ag 2CO3 Ksp 4 x 10 13 in 0 1 M Na CO3 solution b 10 7 c 2x 10 6 a 10 6 142 What is the concentration of Pb 2 when PbSO4 Ksp 1 8 10 8 begins to precipitate fro d 2x 10 7 solution that is 0 0045 M in SO 2

Physical Chemistry
Generala 1 0 x 10 5 What is the molarity of F a 2 3 10 3 3 b 1 0 x 10 c 1 26 x 10 a 6 3 x 10 in a saturated solution of InF3 Ksp 7 9 x 10 10 b 8 3 x 10 3 c 1 0 10 3 d 7 0 x 10 What is the pH of a saturated solution of Cu OH Ksp 2 6 10

Physical Chemistry
General128 The solubility of different sparingly soluble salts are given as under Formula Type Solubility product 4 0 10 20 3 2 x 10 11 2 7 x 10 31 S No 1 2 3 AB A B AB 3 The correct increasing order of solubility is a 1 3 2 b 2 1 3 c 1 2 3 d 3 1 2

Physical Chemistry
GeneralA metal M forms water soluble MSO4 and inert MO MO in aqueous solution forms insoluble M OH 2 soluble in NaOH Metal M is A Be skipped B Mg

Physical Chemistry
GeneralA chemistry student needs 90 0 mL of dimethyl sulfoxide for an experiment By consulting the CRC Handbook of Chemistry and Physics the student discovers 3 that the density of dimethyl sulfoxide is 1 10 g cm Calculate the mass of dimethyl sulfoxide the student should weigh out Be sure your answer has the correct number of significant digits A Q 0x10

Physical Chemistry
GeneralGiven that i H g O2 g H O l AH 286 kJ ii C H g O g 2CO g H O 1 AH 1297 kJ 2 iii C H4 g 302 g 2CO2 g 2H O l AH 1410 kJ The enthalpy change for the process C2H2 g H g C H4 9 is O 173 kJ O 173 kJ O 346 kJ 346 kj

Physical Chemistry
EnergeticsPhotoelectric effect supports quantum nature of light because a the energy of released electron is discrete b the maximum kinetic energy of photoelectrons depends only on the frequency of light and not on its intensity c even when the metal surface is faintly illuminated the photoelectrons leave the surface immediately d electric charge of the photoelectrons is quantised

Physical Chemistry
GeneralA 5 00 mL sample of solution has 2 8 x 104 g of calcium ions The ppm concentration is A B skipped C 18 ppm 56 ppm 2 8 x 10 ppm Class XI Chemistry Mole concept and Stoichiometry 4 for JEE BITSAT Exams Google Chrome e Class XI Chemistry Mole concep

Physical Chemistry
EnergeticsIf an ideal gas expands isothermally from 50 L to 100 L against 2 atm external pressure then values of W AU and Q respectively will be NCERT Pg 166 1 100 L atm zero and 100 L atm 2 50 L atm zero and 50 L atm 3 100 L atm zero and 100 L atm 4 50 L atm zero and 50 L atm

Physical Chemistry
EquilibriumThe equilibrium A g 4B g AB g is attained by mixing equal moles of A Then at equilibrium 1 A B 3 A B 2 A B 4 AB A a one litre

Physical Chemistry
Atomic StructureMatch the parameters of column l with column Il as they are directly proportional Column l A f B T C of 15 E P Q R Column II n Z 1 2 n D S Z Where Frequency f Time period T Energy of nth orbit En radius of nth orbit r Atomic number Z Orbit number n
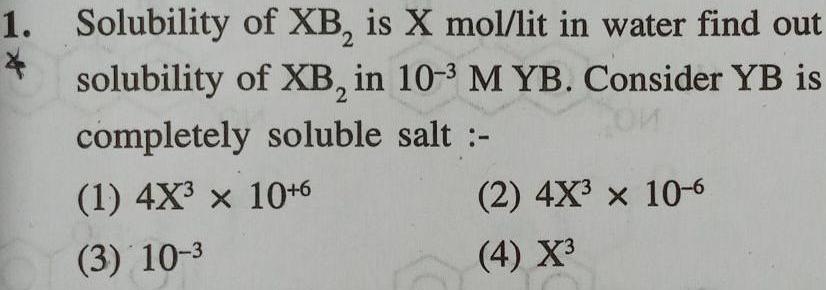
Physical Chemistry
Equilibrium1 Solubility 4 of XB is X mol lit in water find out solubility of XB in 10 3 M YB Consider YB is completely soluble salt 1 4X x 10 6 3 10 3 2 4X x 10 6 4 X

Physical Chemistry
Gaseous and liquid statesWhich reaction is suitable for preparing a Chloroacetic acid 2 Stephen s reaction 1 Hell Volhard Zelinsky reaction 3 Perkin s reaction 4 None of these A mixture of methane and Ethene in a molar ratio of x y has an average molecular mass of The mean molar mass when they are mixed in the molar ratio of y x will be 1 20 2 25 3 24 4 15

Physical Chemistry
SolutionsA solution containing 4 g of a non volatile organic solute per 100 ml was found to have an osmotic pressure equal to 500 cm of mercury at 27 C The molecular weight of solute is A 14 97 B 149 7 lorations C 1697 D 1 497 B If a 6 84 wt vol solution of cane sugar mol wt 342 is isotonic with 1 52 wt vol solution

Physical Chemistry
Solid stateThe radius of a divalent cation A2 is 94 pm and of divalent anion B2 is 146 pm Th compound AB has a Rock salt structure c Antifluorite structure b Zinc blende structure d Caesium chloride like structure

Physical Chemistry
Chemical kineticsIn the metal carbonyls of general formula M CO Which follows EAN rule if M is Ni Fe and Cr the value of x will be respectively 1 6 5 6 3 4 4 5 2 4 5 6 4 4 6 6

Physical Chemistry
Electrochemistryb of sodium benzoate hydrochloric acid sodium chloride are 82 4 426 2 26 53 Sm mol Calculate for benzoic acid A 482 07 Sm mo

Physical Chemistry
Electrochemistryulate the current in mA required to deposit 0 195 g of platinum metal in 5 0 hours from a solution of PtCl2 Atomic weight Pt 195 PUC a 310 Duo b b 3100 5 HO d 5 36 HOW c 21 44

Physical Chemistry
EquilibriumFor the reaction N O g 2NO g 1 2O g calculate the mole fraction of N O g decomposed at a constant volume temperature if the initial pressure is 600 mm Hg the pressure at any time is 960 mm Hg Assume ideal gas behaviour If answer is x then report 10x

Physical Chemistry
General8 Arrange the following in the increasing order of their solubility in n octane based on solute solvent interaction a KCI CH CN CH3OH Cyclohexane 2008 b KCI Cyclohexane CH3OH CH3CN c KCI CH OH CH3CN Cyclohexane 20 d KCI Cyclohexane CH CN CH3CN tulos A O 150910

Physical Chemistry
Chemical kineticsA solution contains Fe2 Fe and I ions This solution was treated with iodine at 35 C E for Fe 0 536 V The Fe is 0 77 V and E for 1 21 favourable redox reaction is AIPMT Mains 2011 1 I will be oxidised to I 2 Fe2 will be oxidised to Fe 3 I will be reduced to I 4 There will be no redox reaction ST

Physical Chemistry
Solutions3 Both temperature and pressure are increased 4 Both temperature and pressure are reduced Aqueous solution of 0 004 M Na SO and 0 01 M glucose are isotonic The percentage degre dissociation of Na So is 1 85 3 60 4 25 Which of the following statements regarding Nitrogen pentoxide is not correct 2 75

Physical Chemistry
General3 The correct match between items of List I and List II is List I A Coloured impurity B Mixture of o nitrophenol and p nitrophenol P Q C Crude Naphtha R D Mixture of glycerol and sugars S 1 A R B S C P D Q 3 A R B P C Q D S List II Steam distillation Fractional distillation Charcoal treatment Distillation under reduced pressure 2 A P B S C R D Q 4 A R B P C S D Q

Physical Chemistry
GeneralPredict whether the equilibrium constants for the following reactions should be greater than 1 or less than 1 a CdI2 s CaF2 s CdF2 s Cal2 s b Cul4 2 aq CuC14 3 aq c NH2 aq H2O 1 NH3 aq OH aq 1 point CuC14 2 aq CuI4 3 aq

Physical Chemistry
General1 2 3 Pressure False Phase diagram Determine the phase of matter or phases occurring at each labeled point on this phase diagram C A E B Temperature F G D 22 4 points What phase or phases of matter is point F Solid and liquid Gas Solid Liquid Solid liquid and gas Supercritical fluid Gas and solid Gas and liquid 00000000 Solid and liquid Supercritical fluid 23 4 points What phase or phases of matter is point G Liquid

Physical Chemistry
Generallg N nagi 4 State the type of PH3 molecule according to the VSEPR theory 5 Considering x axis as the internuclear axis which out of the fo