Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
SolutionsA mixture of 1 60 moles of X gas and 2 70 moles of Y gas are mixed with 6 42 moles of XY gas in a 10 0 L tank at 25 C X Ya 2XY Answer both questions a If the Ke for this reaction is 2 4 x 102 at this temperature is the reaction at equilibrium b If not what is the direction of the net reaction

Physical Chemistry
Chemical Bondingliberated The gas is 1 CL 3 N 4 H C H O is a carbonyl compound The number of structural isomers possible for this molecula formula are 2 NH 1 5 2 8 3 6 4 7 In the reaction 44 2B 3C A B C what will be the number moles of product formed

Physical Chemistry
Electrochemistry4 Fi 43 Deduced from the following E values of half cells what combination of two half cells would result in a cell with the largest potential i A A e ii B2 e B iii C e C iv DD 2e 1 i and iii 3 ii and iv E 1 5 V E 2 1 V E 0 5 V E 1 5 V 2 i and ii 4 iii and iv

Physical Chemistry
Atomic StructureAssuming Heisenberg Uncertainity Principle to be true what could be the minimum uncertainty in de brogli wavelength of a moving electron accelerated by Potential Difference of 6 V whose uncertainty in position 7 22 A 6 25 A is n m B 6 A C 0 625 A D 0 3125 A

Physical Chemistry
GeneralTE TE IE IE Element S 796 1583 3238 4362 The values of dissociation constant of some bases are given below Which is the weakest base 1 1 8x10 2 4 8x10 0 3 7 2x10 4 7 07 10

Physical Chemistry
General2 HCl 1 aq MgCl aq The initial masses of the uncombined reactants appear below Mg Mass of Magnesium Mg Mass of Hydrochloric Acid HCI 99 1 0 H 51 The reactants are combined and the final mass of the system is determined If the final mass of the system is 120g what was the percent yield of the reaction 35 48 g 73 g

Physical Chemistry
General3 CH CH CH CH CH C CH CH CH 4 CH CH CH CH CH C CH CH CH Which of the following is the disproportionation redox reaction 1 2CH COOH is 2 2CH CHO NG I 3 2CH COCH 4 2HCHO 50 NO g H O Determine the stability order of given carbocations

Physical Chemistry
General20 ml solution contains 0 1 M NH4Cl and 0 01 M NH4OH By adding which one its pH will not change Addition of 1ml water O Addition of 5ml 0 1 M NH4Cl O Addition of 5ml 0 1 M NH4OH O Addition of 10ml 0 1M NH4Cl

Physical Chemistry
GeneralUse the balanced equation to answer the following question 6CUNO3 Al2 SO4 3 3Cu2SO4 2AI NO3 3 Molar mass of CUNO3 125 56 g mol Molar mass of Al NO3 3 213 01 g mol How many grams of copper 1 nitrate CUNO3 are required to produce 44 0 grams of aluminum nitrate Al NO3 3 77 81 g 155 62 g 24 88 g

Physical Chemistry
GeneralDraw the complete mechanism for the radical reaction shown light CH CI Arrow pushing Instructions 3 Use curved arrows to show the mechanism of the initiation step below Make the ends of your arrows specify the origin and destination of reorganizing electrons Sight 00 Br m light H Homolytic cleavage of Br gives two Br radicals light 20 Homolytic cleavage of Br gives two Br radicals 2 Br Arrow pushing Instructions points Delete CH CIBr Correct b Use curved arrows to show the mechanism of the propagation step below Make the ends of your arrows specify the origin and destination of reorganizing electrons Correct Previous Next Email instructor
Physical Chemistry
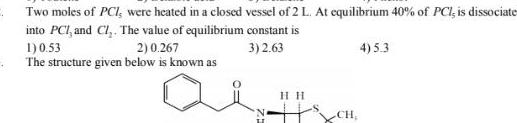
EquilibriumTwo moles of PCI were heated in a closed vessel of 2 L At equilibrium 40 of PCI is dissociate into PCI and Cl The value of equilibrium constant is 3 2 63 1 0 53 2 0 267 The structure given below is known as au HIH CH 4 5 3

Physical Chemistry
EquilibriumFind the concentration of manomeric dichloroacetic acid in a CCl4 solution which contains 0 0129 g of the acid in 100 ml of solution The dissociation constant of the dimeric acid is 5 0 10 4 Assume that the acids are unionized in CCl4 solution a 5 0 x 104 M c 1 0 10 M b 2 5 x 104 M d 3 9 x 104 M

Physical Chemistry
General1 5 2 8 4 In the reaction 4 4 2B 3C A B C what will be the number moles of product formed starting from one mole of A 0 6 moles of B and 0 72 moles of C 1 0 25 2 0 3 3 0 24 4 2 32 The solubility of AgCl s with solubility product 1 6 10 in 0 1 M NaCl solution would be 1 1 26 10 M 3 1 6 10 M 4 1 26 10 M 2 1 6 10 M

Physical Chemistry
GeneralWhich one is the wrong statement 1 The uncertainty principle is AE x At h 4t 2 Half filled and fully filled orbitals have greater stability due to greater exchange energy greater symmetry and more balanced arrangement 3 The energy of 2s orbital is less than the energy of 2p orbital in case of Hydrogen like atoms h 4 de Broglie s wavelength is given by A my where m mass of the particle v velocity of the particle

Physical Chemistry
Solutions7 How many ions are produced from the complex Co NH3 6 Cl2 in solution i 6 ii 4 iii 3 iv 2 VIC ICON Lio

Physical Chemistry
SolutionsSelect the statements that describe the result when a nonvolatile solute is dissolved in a liqui solvent Check all that apply The boiling point of the solution is lower than that of pure solvent The boiling point of the solution is higher than the boiling point of pure solvent The amount that the boiling point increases is dependent only on the concentration of dissolved particles The amount that the boiling point increases is dependent on the identity of the solute Do you know the answer

Physical Chemistry
Gaseous and liquid states6 Estim of oxygen nitrogen water vapour and other constituents in a room of capacity 25 0 m at a temperature of 27 C and 1 atm pressure Solution Volume of the room V 25 0 m Temperature of the room T 27 C 300 K Pressure in the room P 1 atm 1 x 1 013 x 105 Pa The ideal gas equation relating pressure P Volume V and absolute temperature T can be written as PV KBNT Where KB is Boltzmann constant 1 38 x 10 23 m kg s K 1 N is the number of air molecules in the room Therefore N PV KBT 1 013 x 105 x 25 1 38 x 10 23 x 300 We get 6 11 x 1026 molecules Hence the total number of air molecules in the given room is 6 11 x 1026

Physical Chemistry
Atomic Structure5 E 313 6 n2 kcal mole If the value of E 34 84 kcal mole to which value does n correspond 1 4 3 2 2 3 4 1
Physical Chemistry
GeneralAt a constant pressure P the plot of volume V as a function of temperature T for 2 moles of an ideal gas gives a straight line with a slope 0 328 L K value of P in atm is closest to Gas constant R 0 0821L atm mol K A 0 25 0 5

Physical Chemistry
SolutionsThe molar conductivities at infinite dilution of barium chloride sulphuric acid and hydrochloric acid are 280 860 and 426 S cm mol 1 respectively The molar conductivity at infinite dilution of barium sulphate is S cm mol 1 Round off to the Nearest Integer ven 288 er

Physical Chemistry
General3 Calculate the difference between heat of combustion of carbon monoxide gas at constant pressure and at constant volume at 27 C R 2 cal K mol A 54 cal B 600 cal C 300 cal D 27 cal 4 The conductivity of an electrolytic solution decreases on dilution due to

Physical Chemistry
General56 57 The configuration of the compound H C 1 R I CH Cl is 2 S 3 E 4 Z The first ionization energies of magnesium and aluminium are respectively 1 7 64 5 98 2 7 64 7 64 3 5 98 7 64 4 5 98 5 98 given by

Physical Chemistry
Atomic Structure3 The ionization energy of the electron in the lowest orbit of hydrogen atom is 13 6 eV The energies required in eV to remove electron from three lowest orbits of hydrogen atom are 1 13 6 6 8 8 4 2 13 6 10 2 3 4 4 13 6 3 4 1 51 3 13 6 27 2 40 8

Physical Chemistry
Chemical kinetics25 At a certain temperature the half life periods for the catalytic decomposition of NH were found to be as follows Pressue mm Hg 50 100 200 Half life period hrs 3 52 1 76 0 88 What will be the pressure when the half life period 1 5 hours

Physical Chemistry
GeneralA block A of mass 2m is hanging from a vertical massless spring of spring constant k and is in equilibrium Another block B of mass m strikes the block A with velocity u and sticks to it as shown in the figure The magnitude of the acceleration of the combined system of the blocks just after the collision is 8 000000 2m A 3 o 4 zem

Physical Chemistry
Atomic StructureAccording to Bohr s theory En Total energy V Potential energy Match the following C D K Kinetic energy n Column I A V K n B If radius of nth orbit x EX x 2 x Z y ba Radius of nth orbit Angular momentum in lowest orbital p 0 9 1 2006 6M Column II S r 2 E A

Physical Chemistry
General1 00 g of BaCl2 is treated with excess of aqueous AgNO3 and all chlorine is recovered as 1 38 g of AgCl What is the atomic weight of BA Cl 35 5 Ag 108 A 137 B 172 5 C 33 D 68 5

Physical Chemistry
SolutionsArrange the following aqueous solutions in order of increasing boiling poin Assume all dissociate completely in solution and have the same Kb Highest boiling point 1 2 3 A 0 250 M Na2SO4 aq 0 300 M MgSO4 aq 0 100 M NaCl aq 0 100 M NH3 aq

Physical Chemistry
GeneralAn aqueous solution contains a mixture of Ca NO and Al NO A sample of this aq solution is 2M in Ca NO3 and 3m in AI NO What should be the density of solution such that the molality and molarity of NO ion becomes numerically same Molar masses Ca NO 164 g mol Al NO 213 g mol A 1 0 gm ml B 1 967 gm ml C 1 310 gm ml

Physical Chemistry
Generalven 3 er A xenon compound A upon partial hydrolysis gives XeO F The number of lone pair of electrons present in compound A is Round off to the Nearest Integer

Physical Chemistry
Atomic Structure1 163 167 3 168 2 171 2 172 3 175 3 176 4 179 3 180 4 183 3 184 4 187 4 188 3 169 4 173 2 177 3 181 4 185 3 170 2 174 3 178 1 1 4s 2 4p The shape of the orbital with I 3 4d a mon 1 Angular 2 Qantum nur 3 Dual nature 182 3 4 Black bod 186 2 200 In the plots the hydrog QUANTUM MECHANICAL MODEL OF ATOM 9 The designation of a subshell with n 4 and 3 is 4 4f peaks are 1 3 201 Which of for 1 y mus 2 y mu

Physical Chemistry
Electrochemistry6 THE DIAGRAM BELOW SHOWS ELECTROLYSIS OFACIDIFIED WATER 0 KIS di Nonieti fena cons ELECTROLYSIS OF WATER SOUTE 1 4N BATTHY HOFMANN VOLTAMETER HYDR A NAME THE APPARATUS USED FOR ELECTROLYSIS B NAME THE ELECTROLYTE USED
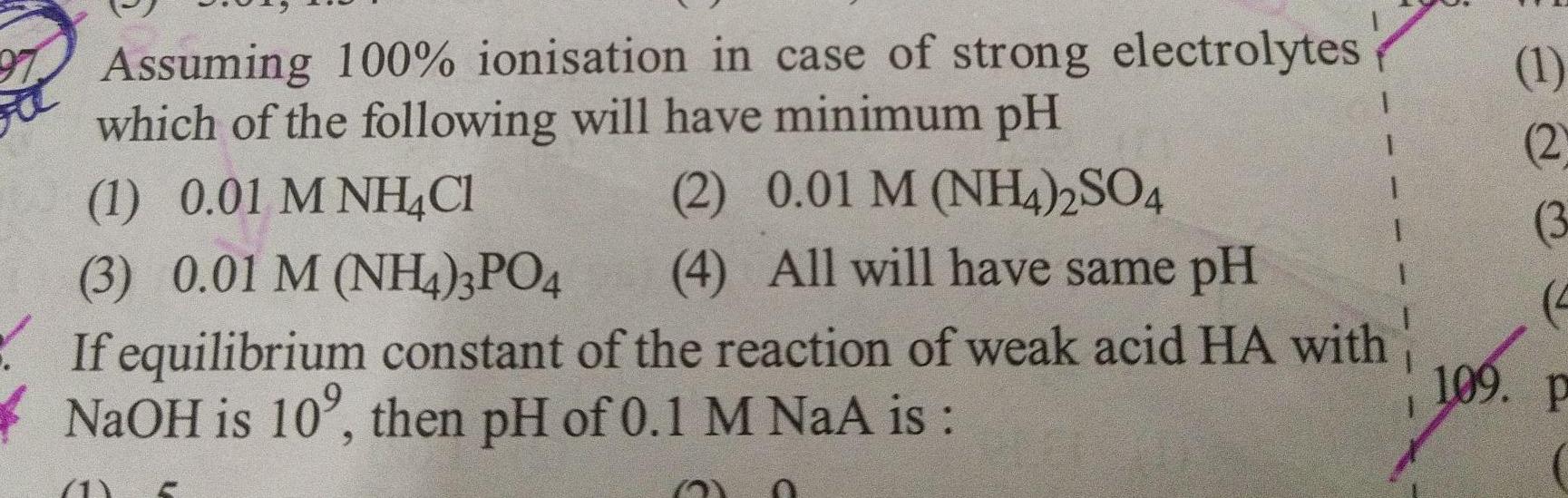
Physical Chemistry
SolutionsAssuming 100 ionisation in case of strong electrolytes which of the following will have minimum pH 1 0 01 M NH4Cl 2 0 01 M NH4 2SO4 3 0 01 M NH4 3PO4 4 All will have same pH If equilibrium constant of the reaction of weak acid HA with NaOH is 10 then pH of 0 1 M NaA is 1 2 3 4 109 P 1

Physical Chemistry
Atomic StructureFor H spectrum electron transition takes place from n 5 ton 2 then emitted wavelength of photon is 434 nm The wave length of photon in electron transition from n 4 to n 2 will be A 1 586 16 nm 31x8 2 48 608 nm 3 486 nm

Physical Chemistry
SolutionsS E 22 M m m nim n m 20x 80 00 40x 2000 20x600 30x20000 40 x 30 30x60 2X4x4x 12 36 108 x 10 6 12 18 X 105 43 333 gram Answer 23 46 Answer rated as Satisfied Contact Tutor option is selected sir why did we have square in nume dear student this is actually a formula for polymers four kind of average molecular weight number average weight average viscosity average atc are thoro ach have different

Physical Chemistry
Chemical Bonding5 solution 1 2 2 1 3 13 4 12 The correct order of increasing thermal stability of the given compounds is 1 HF II HBr III HC IV HI 1 I II III IV 2 IV II III 1 4 II IV 1 III 3 IV II I III Br

Physical Chemistry
Equilibrium20 Solubility products of M OH and M OH are 10 23 and 10 14 respectively Which will be precipitated first on adding NH OH if M 2 and M 3 both the ions are in solution 2 M 3 4 Precipitation will not take place 1 M 2 3 Both M 2 and M together Ans 2

Physical Chemistry
Gaseous and liquid statesThe vapour pressure of pure liquid solvent A is 0 80 atm When a nonvolatile substance B is added to the solvent its vapour pressure drops to 0 60 atm What is the mole fraction of component B in the solution

Physical Chemistry
Equilibriumc 1000 ppm d 1 0 gm of pure calcium carbonate was found to require 50 ml of dilute HCl for complete reaction The strength of the HCI solution is given by CPMT 1986 a 4 N c 0 4 N b 2 N d 0 2 N of an

Physical Chemistry
Chemical BondingUsing graph concentration of reactants and products as a function of time for the reaction A33A The time t corresponds to 1 t conc 2 t A k 2 303 t time A e lo C

Physical Chemistry
Atomic StructureAn incident UV light having wave length is incident on metal surface and the ejected electron has the velocity of V Another UV light having wave length is incident on the same metal surface ejects electron having velocity V then V2 V is 1 2hc 1 111 me2 2 2hc 2 2hc 1 1 me 2ho 2 A 2 2

Physical Chemistry
GeneralAn element occurs in BCC structure with a edge length of 288 pm The density of the element is 7 2 gm cm How many atoms of the elemen does 208 g of the element contain 1 24 16 x 1022 2 24 16 x 1023 3 24 16 x 1024 A

Physical Chemistry
General4 i Suppose 2000 marbles glass balls are moving randomly on a floor which is 10 ft x 10 ft If the diameter of each marble is 1 inch and their velocity is 5 ft s derive formulas for and then calculate the mean free path the number of collisions per minute made by each marble and the total number of collisions per minute

Physical Chemistry
Equilibriumn analytical chemist is titrating 194 2 ml of a 0 4800M solution of piperidine C H NH with a 0 6500M solution of HIO The pK of piperidine is 2 89 alculate the pH of the base solution after the chemist has added 35 2 ml of the HIO solution to it ote for advanced students you may assume the final volume equals the initial volume of the solution plus the volume of HIO solution added ound your answer to 2 decimal places

Physical Chemistry
Gaseous and liquid states3 i Based on the Barometric distribution law estimate the height at which partial pressure of oxygen is half of its sea level value at 25 C assume with changing height temperature does not change

Physical Chemistry
GeneralAmong ligands NH3 en CO and CN the correct field strength order is A skipped B NH3 en GN CO D CO NH3 CN en CO NH en CN NH3 en CO CN

Physical Chemistry
EquilibriumWhich of the following is not true for solid liquid equili 1 It can be established at any given temperature 2 The mass of solid does not change with time 3 The mass of liquid does not change with time There is no exchange of heat between the system and its surroundin

Physical Chemistry
General62 63 Which among the following depicts the correct order of acidity 1 CH CH CH CH CH CH C CH CH CH 2 CH CH CH C CH CH CH CH CH 3 CH CH CH CH CH C CH CH CH 4 CH CH CH CH CH C CH CH CH Which of the following is the disproportionation redox reaction

Physical Chemistry
Equilibriumven 2 er The solubility of CdSO in water is 8 0 x 10 4 mol L 1 Its solubility in 0 01 M H SO4 solution x10 6 mol L 1 Round off to the Nearest Integer is Assume that solubility is much less than 0 01 M

Physical Chemistry
General83 84 A B CH CH CH 2 A C D B D CH CH CH 1 C B D A 4 C B A D The paramagnetic species is 1 SiO 2 Tio 3 BaO 4 KO In which of the following compounds nitrogen exhibits highest oxidation state C CH CH 3 A C B D