Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Equilibriuma 8 0 x 10 18 139 What is the minimum pH necessary to cause a precipitate of Pb OH 2 Ksp 1 2 x 10 to c 8 0 x 10 17 d 8 0 x 10 14 b 8 0 x 10 form in a 0 12 M PbCl solution a 12 4 b 10 8 40 Which of the following would incre a Add 1 15 sp c 12 0 d 11 1

Physical Chemistry
Solutions1 1 1 1 2 1 2 3 3 3 2 1 01 3 1 At a constant temperature which of the following aqueous solutions will have the maximum vapour pressure Mol wt NaCl 58 5 H SO 98 0 g mol 3 1 molal H SO aq 1 1 molal NaCl aq 2 1 molar NaCl aq 4 1 molar H SO aq When two reactants A and B are mixed to give products C and D the reaction quotient Q at

Physical Chemistry
Equilibriuma 1 0 b 1 29 c 1 512 d 4 65 134 What is the molarity of F ions in a saturated solution of BaF2 Ksp 1 0 10 6 a 1 0 x 10 2 d 6 3 10 b 1 0 x 10 c 1 26 x 10 2 135 What is the molarity of F in a saturated solution of InF3 Ksp 7 9 x 10 10

Physical Chemistry
GeneralWhich of the following statements is are INCORRECT All spectral lines belonging to Balmer series in hydrogen spectrum lie in visible region If a light of frequency v falls on a metal surface having work function hvo photoelectric effect will take place only if vs Vo The number of photoelectrons ejected from a metal surface in photoelectric effect depends upon the frequency of incident radiations 4 The series limit wavelength of Balmer series of H atom is R where R is Rydberg s constant

Physical Chemistry
General21 For the gas phase reaction PCI 9 PCL g Cl g which of the following conditions is correct AIPMT Prelims 2008 1 AH 0 and AS 0 2 AH 0 and AS 0 3 AH 0 and AS 0 4 AH0 and AS 0

Physical Chemistry
Chemical kineticsConsider the reaction 2B C AH 15 kcal The energy of activation of backward reaction is 20 kcal mol In presence of catalyst the energy of activation of forward reaction is 3 kcal mol At 400 K the catalyst causes the rate of the forward reaction to increase by the number of times equal to 1 e 2 e 3 e2 4 201

Physical Chemistry
Equilibrium14 Following reaction occurring in an automobile 16CO g 18H O g The 2CH 9 250 g sign of AH AS and AG would be 1 2 3 4 15 When 5 litres of a gas mixture of methane and

Physical Chemistry
General4 DCM and H O will make turbid colloidal mixture Hard water can block radiators due to the formation of 1 Insoluble calcium and Magnesium salts 3 Insoluble Phosphate salts 2 Insoluble Sodium salts 4 Insoluble Potassium salts Which one of the two lodine atoms will be more reactive in the SN and SN reaction

Physical Chemistry
GeneralWrite a short story in about 100 120 on the basis of the following inputs given in th old lady alone maid left two robbers in early 20s robbery woman stabbed in hospital assailants expected to be caught

Physical Chemistry
GeneralWhich of the following statement is incorrect about silicones O R SiCl RSiCl3 are monomers of silicones O Silicones are hydrophobic O R3SiCl is used to close polymeric chain O Polymers of SiO4

Physical Chemistry
SolutionsAn azeotropic solution of two liquids has boiling point lower than either of them when i 1 shows negative deviation from Raoult s law 2 shows no deviation from Raoult s law 3 shows negative deviation from Raoult s law 4 is saturated


Physical Chemistry
GeneralA permanganate solution is prepared by dissolving 20 0123 g KMnO4 in 500 mL of distille water and boiled for 1 hour to remove any organic material Following sintered glas filtration the solution is quantitatively transferred to a 1 0 L volumetric flask and diluted volume with distilled water The permanganate solution was titrated against 0 1023 oxalic acid prepared in sulfuric acid solution A 50 00 mL aliquot of oxalic acid solut required 16 68 mL of permanganate solution A titration blank required 0 04 mL permanganate What is the permanganate molarity 5 H C O4 2 MnO4 6H S 10 CO 2 Mn 8 H O

Physical Chemistry
General22 1 g sample of chalk is completely neutralised by 100 ml 0 04N HCI solution w w CaCO3 in the sample is are 1 20 3 60 2 40 4 80

Physical Chemistry
Electrochemistry2 Cu s Cut2 1 M Zn2 1 M Zn s Cist A cell represented above should have emf 1 Positive E 2 Negative 3 Zero 4 Cannot be predicted

Physical Chemistry
Gaseous and liquid states19 From the following bond energies H H bond energy 431 37 kJ mol 1 C C bond energy 606 10 kJ mol C C bond energy 336 49 kJ mol C H bond energy 410 50 kJ mol 1 Enthalpy for the reaction HH C H HH C C H HH HH will be 1 243 6 kJ mol 1 2 120 0 kJ mol 3 553 0 kJ mol HH AIPMT Prelims 2009

Physical Chemistry
ElectrochemistryWhich of the following is incorrect O Am NaCl Am NaBr Am KC1 Am KBr A H O A HCl NaOH Am NaCl 0 Am NaCl Am KCl AM NaBr Am KBr Am Nal Am NaBr Am NaBr Am KBr

Physical Chemistry
Gaseous and liquid states0 Which of the following pair of gases will diffuse at the same rate through a porous plug a CO NO c NO2 CO2 b NO C H6 d NH3 PH3

Physical Chemistry
SolutionsLatent heat of vaporization of water is 9 72 kcal mol at 373 15 K calculate molal boiling point elevation constant of water a 5 2 c 52 2 b 0 052 d 0 52

Physical Chemistry
Atomic StructureMr Santa has to decode a number ABCDEF where each alphabet is represented by a single digit Suppose an orbital whose radial wave function is represented as Yk ek 5kr 2 6k From the following information given about each alphabet then write down the answers in the form of ABCDEF for above orbital Info A Value of n where n is principal quantum number Info B Number of angular nodes Info C Azimuthal quantum number of subshell to orbital belongs Info D Number of subshells having energy between n 5 s to n 5 p where n is principal quantum number Info E Orbital angular momentum of given orbital Info F Radial distance of the spherical node which is farthest from the nucleus Assuming k3 1

Physical Chemistry
Chemical BondingWhat is the dominant intermolecular force or bon that must be overcome in converting liquid CH O to a gas 1 London dispersion force 2 Hydrogen bonding 3 Dipole dipole interaction 4 Convalent bonds

Physical Chemistry
Equilibrium1 1 15 kJ 2 15 1 kJ 3 49 1 kJ 4 1511 KJ For a reaction equilibrium NO 8 2NO 8 the concentration of NO and NO a equilibrium are 4 8 10 and 1 2x10 mol L respectively The value of K for the reaction is 2 3 3x10 mol L 3 3x10 mol L 4 3 3x10 mol L 1 3x10 mol L An azeotropic solution of two liquids has boiling point lower than either of them when it 1 shows negative deviation from Raoult s law

Physical Chemistry
Equilibriumc K 2 C CH3COOH is 2 0 ionised K 1 8x 10 hence its molar concentration is a 0 045 M c 3 6 x 10 5 M b 0 02 M d 0 090 M unak acid is 1 at 1 M hence ionisation

Physical Chemistry
Electrochemistryom the following equivalent conductances at infinite dilution A for Ba OH 288 8 ohm cm equivalent Ao for BaCl 120 3 ohm cm equivalent Ao for NH4C1 129 8 ohm cm equivalent culate Ao for NH OH Ans 238 3 ohm C

Physical Chemistry
EquilibriumJeq The equilibrium constant for the reaction Kc 9 4 2X s Y g 2Z g What would be the concentration of Y at equilibrium if 3 0 mole of and 3 0 mole of Z are present in 2 litre vessel at equilibrium
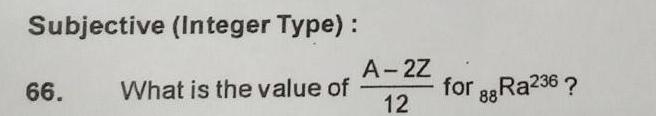
Physical Chemistry
Atomic StructureSubjective Integer Type 66 What is the value of A 2Z 12 for Ra236 88

Physical Chemistry
Equilibrium3 a iv b i c d ii 4 a i b i c iv d ii 5 Three moles of an ideal gas expander spontaneously into vacuum The work done will be AIPMT Mains 2010 2 3 Joules 4 Zero 1 Infinite 3 9 Joules

Physical Chemistry
Solutions3 At a temperature under high pressure Kw H O 1x10 10 A solution of pH 5 4 under these conditions is said to b a acidic c neutral b basic d amphoteric 12

Physical Chemistry
GeneralProblem 1 1 Mass of an atom of oxygen in gram is 26 56896 x 10 24 g What is the atomic mass of oxygen in u

Physical Chemistry
GeneralChoose the disproportionation reaction from the following a XeF4 H O b Cl NaOH c P4 NaOH aq d XeF2 H O O a b Ob c O b c and d O a b c

Physical Chemistry
General3 Insoluble Phosphate salts 4 Insoluble Potassium salts Which one of the two lodine atoms will be more reactive in the SN and SN reaction 1 A will be faster in SN reaction but slower in SN 2 A will be faster both in SN and SN reaction 3 A and B will be equally reactive 4 will be factor in both SN and SN reaction

Physical Chemistry
EnergeticsChoose the correct value of entropy change for the following phase change H O 1 373 K 1 atm H O g 373 K 1atm Standard enthalpy of formation of liquid water and gaseous water are 285 8 kJ mol an 241 8 kJ mol respectively

Physical Chemistry
EnergeticsIf this reaction releases 599 kJ of energy how many grams of H O are formed Show the conversions required to solve this problem 2H g O g 2H O g 599 kJ x 18 02 H O 2 02 H Answer Bank 1 mol 0 4841J AH 484 kJ 1 mol H 2 mol H H O

Physical Chemistry
EnergeticsIf the enthalpy change for the transition of liquid water to steam is 30 kJ mot at 27 C the entropy change for the process would be AIPMT Prelims 2011 1 100 J mol 1 K 1 2 10 J mol K 1 3 1 0 J mol K 4 0 1 J mol K Enthalpy change for the reaction 4 H2H

Physical Chemistry
Generala 12 4 D 140 Which of the following would increase the solubility of Pb OH 2 a Add hydrochloric acid b Add a solution of Pb NO3 2 c Add a solution of NaOH d None of the above the solubility of a compound is constant at constant temperat

Physical Chemistry
Surface chemistryWhich of the following is correct statement regarding zeolite O Size of pores is approximately 300nm 700nm O Zeolite is 2 D silicate O Zeolite is sodium alumino silicate O Zeolite is used as catalyst in hydrolysis of ester FADER 27

Physical Chemistry
EquilibriumIf Ksp of Ag CO3 is 8 x 10 12 the molar solubility of Ag CO3 in 0 1 M AgNO3 is h A 00 C 8x 10 12 p M 8x 10 10 M 8 10 M D 8 10 13 M

Physical Chemistry
GeneralQuestion 4 The value of kp for the reaction at 27 C Br e Cl g 2BrCl g is 1 atm At equilibrium in a closed container partial pressure of BrCl gas is 0 1 atm and at this temperature the vapour pressure of Br2 e is also 0 1 atm Then what will be minimum moles of Br e to be added to 1 mole of Cl initially to get above equilibrium situation Options 10 6 a moles b moles 15 Vedantu YOUR PERSONAL TEACHER ONL

Physical Chemistry
Atomic StructureIn face centred cubic lattice tetrahedral voids are present at O each face diagonal O each cube diagonal O body centre Orach nin

Physical Chemistry
Chemical BondingA B 3 and the percentage error in the measurements of A B C and D are 4 2 3 and 1 respectively CD3 2 Example 12 Find the relative error in Z if Z
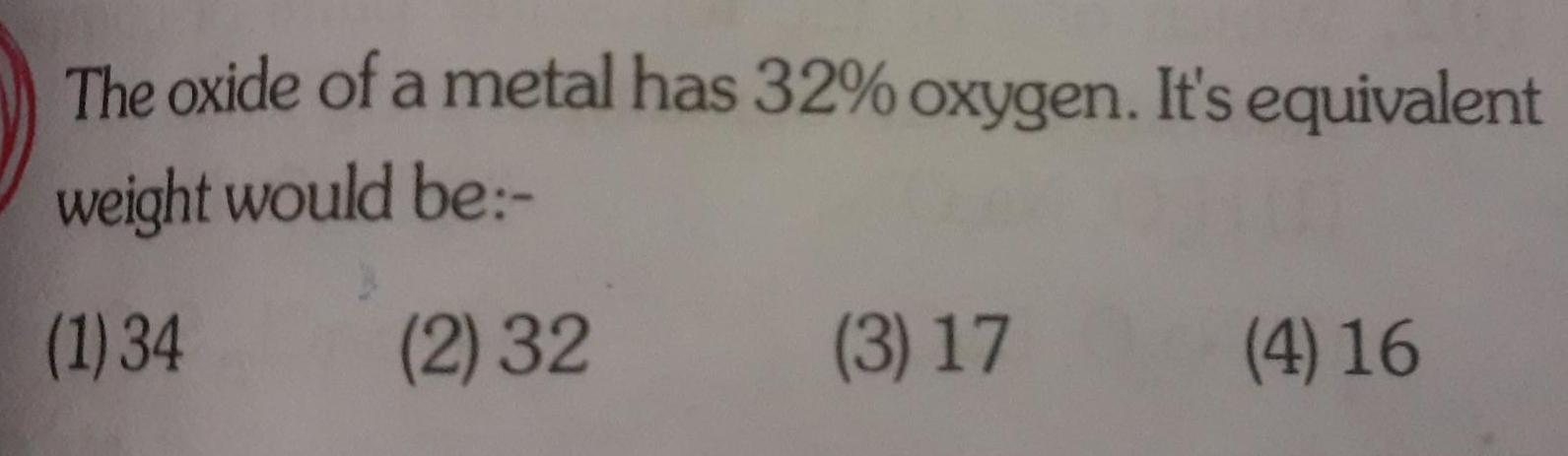
Physical Chemistry
GeneralThe oxide of a metal has 32 oxygen It s equivalent weight would be 1 34 2 32 3 17 4 16

Physical Chemistry
GeneralAmongst the compounds given the one that would form a brilliant coloured dye on treatment with NaNO in dil HCI followed by addition to an alkaline solution of B naphthol is 1 N CH 2 NHCH 3 H C NH CH NH

Physical Chemistry
Gaseous and liquid statesConsider an ideal gas contained in a vessel If the intermolecular attraction suddenly begins to act which of the following will happen 1 The pressure decreases 2 The pressure increases 3 The pressure remains unchanged 4

Physical Chemistry
Gaseous and liquid statesThe number of ZnS formula units present in a unit cell of ZnS are 1 2 2 1 3 4 4 8

Physical Chemistry
Atomic StructureOn absorbing light of wavelength 3800 bromine molecule undergoes dissociation and form atoms The kinetic energy of one bromine atom assuming that one quantum of radiation is absorbed by each molecule would be Bond energy of Br 190 kJ mol 1 1 04 x 10 19 J 2 2 08 x 10 19 J 4 6 25 x 104 J 3 1 25 x 10 5 J

Physical Chemistry
Electrochemistry4 trans Co en Cl 1 cis PI NH CI 2 trans PI NH CL1 3 cis Co en CLI How much chlorine will be liberated on passing one ampere current for 30 minutes throught NaCl solution 1 0 66 mole 2 0 33 mole 3 0 66 g 4 0 33 g The heat of dissociation of benzene in isolated gaseous atoms is 5335 kJ mol The bond enthalpies of C C C C and C H bonds are 347 3 615 and 416 2 kJ respectively Ma

Physical Chemistry
GeneralA catalyst cannot affect A Product B Rate of reaction C Reactant D Both A B The lattice site in a pure crystal cannot be occupied by A Molecule B Ion FI

Physical Chemistry
SolutionsThe molality of a sulphuric acid solution is 0 2 mol kg The total weight of the solution containing 1 mol H SO4 O 1000g O 1098 g O 5000 g O 5098 g

Physical Chemistry
Equilibrium16 For the gaseous equilibrium reaction A B 2C D the value of equilibrium constant at 300 K is 50 mol lit Calculate K for the reaction at this temperature Ans 1230 atmosphere 7 For the equilibrium gaseous reaction

Physical Chemistry
Chemical kinetics3 The entropy of vaporization of benzene is 85 JK mol When 117g benzene vaporizes at it s normal boiling point the entropy change of surrounding is 1 85 JK 2 85 x 1 5 JK 3 85 1 5 JK 4 None of these