Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.


Physical Chemistry
GeneralThe enthalpy of neutralisation of HCI and NaOH is 57 kJ mol The heat evolved at constant pressure in kJ when 0 5 mole of H SO4 react with 0 75 mole of NaOH is equal to O42 75 52 50 20 50

Physical Chemistry
GeneralThe weight of 350 ml of a diatomic gas at 0 C and 1 atm pressure is lg The weight of one atom is in gram Assume gases are ideal A 16 NA B 32 NA C 16NA D 32NA

Physical Chemistry
General34 The vapour density of a mixture of NO and N O4 is 39 at 25 C What is the mass of NO present in 100 g of the mixture mol 2 8 4 960 60 1 17 9 g g 2 222 4 66 4 g

Physical Chemistry
EnergeticsIf X X2 and x3 are enthalpies of H H O O and O H bonds respectively and x4 is the enthalpy of vaporization of water estimate the standard enthalpy of combustion of hydrogen x2 O x 2x3 x4 2x3 X4 x2 O x 2 2 x3 x4 x1 X2 Ox 22 x2 O x 22 2 x3 X4

Physical Chemistry
EquilibriumAt a particular temperature the following reaction is carried out with 1 mol of A g and 1 mol of B g in a closed vessel A g 4B g AB g of AB g be higher Will the equilibrium concentration than that of A g

Physical Chemistry
Atomic StructureIn compounds of type ECI where E is B P As or Bi The angles CI E CI for different E are in order 2 B P As Bi 3 B P As Bi 4 B P As Bi 1 B P As Bi For the reaction Cl 211 2C the initial concentration of I was 0 20 mol I and the concentration after 20 min was 0 18 mol Z Then the rate of formation of 1 in mol L min

Physical Chemistry
EnergeticsAt 27 C one mole of an ideal gas is compressed isothermally and reversibly from a pressure of 2 atm to 10 atm The values of AE and q are R 2 and log 5 0 698 1 0 965 84 Cal 3 865 58 Cal 865 58 Cal The angular momentum of an electron in a Bohr s orbit of He is 2 965 84 Cal 865 58 Cal 4 0 865 58 Cal 3 1652x10 kg m sec What is

Physical Chemistry
Generalc Al SO4 3 14 If Ksp BaSO4 is 1 1x10 10 then in which of the following cases BaSO4 is precipitate out a 100 mL of 4 x 10 M BaCl 300 mL of 6 0 x 10 4 M Na2SO4 b 100 mL of 4 x 10 4M BaCl 300 mL of 6 0 10 M Na2SO4 c 300 mL of 4 x 10 M BaCl 100 mL of 6 0 x 10 M Na SO4 d All of the abou

Physical Chemistry
EquilibriumEquilibrium constants for the reversible reactions 2H O g 2H g O g and 2CO g 2CO g O g are 2 1 x 10 13 1 4 x 10 12 mol lit at a given temperature Cal equilibrium constant for the reaction H g CO g H O g CO g at the same tempe Ans T

Physical Chemistry
Chemical BondingChoose incorrect statement regarding alkaline earth metal compounds Solubility of hydroxides increases down the group Solubility of carbonates decreases down the group Tendency of nitrates to form hydrates increases down the group Tendency of halides to form hydrates decreases down the group

Physical Chemistry
GeneralThe enthalpy of fusion of water is 1 435 kcal m The molar entropy change for the melting of ice C is AIPMT Prelims 201 1 5 260 cal mol K 2 0 526 cal mol K 3 10 52 cal mol K 4 21 04 cal mol K

Physical Chemistry
Nuclear chemistryJLLUSTRATION 4 If the atomic masses of lithium helium and proton are 7 01823 amu 4 00387 amu and 1 00815 amu respectively calculate the energy that will be evolved in the reaction Li7 H1 2He4 energy Given that 1 amu 931 MeV

Physical Chemistry
EnergeticsGiven that the enthalpy of formation of CO g H O l and C H6 g are 393 5 kJ 286 kJ and 84 kJ respectively The enthalpy of combustion of ethane is O 1410 kJ O 1297 kJ O 1561 kJ 1561 kJ

Physical Chemistry
GeneralQ 7 Which of the following coordination entity should be expected to absorb light of lowest frequency Cr en CrCl6 Cr NH3 Incorrect 1 002 4 Cr CN

Physical Chemistry
Solutions14 What is the pH of 10 6 M HCI at 25 C NCERT 1 6 3 60H eil 2 7 4 7 beng ins

Physical Chemistry
GeneralWhich of the following vitamin has isoprene units in its structure A Vitamin A B Vitamin C C Vitamin B

Physical Chemistry
Gaseous and liquid statescompressibility factor is changed from 1 2 to 1 6 respectively Calculate the final volume of the gas Reduced temperature for benzene is 0 7277 and its reduced volume is 0 40 Calculate the reduced pressure of benzene

Physical Chemistry
Gaseous and liquid states7 At what temperature is the rms speed of hydrogen molecules the same as that of oxygen molecules at 1327 C 1 173 K 3 400 K 2 100 K 4 523 K

Physical Chemistry
Atomic Structure3 865 58 Cal 865 58 Cal 4 0 865 58 Cal The angular momentum of an electron in a Bohr s orbit of He is 3 1652x10 kg m sec What is the wave number is terms of Rydberg constant R of the spectral line emitted when an electron falls from this level to the first excited state Use h 6 626x10 Js 1 3R 2 SR 3R 3 31 8R 9

Physical Chemistry
Gaseous and liquid statesThe internal energy change in the conversion of 1 0 mole of the calcite form of CaCO3 to the aragonite form is 0 21 kJ Calculate the enthalpy change when the pressure is 1 0 bar given that the densities of the solids are 2 71 g cm and 2 93 g cm respectively O 229 72J mol 1 O 249 12 J mol 1 O 289 82 J mol 1 O 209 72 J mol 1
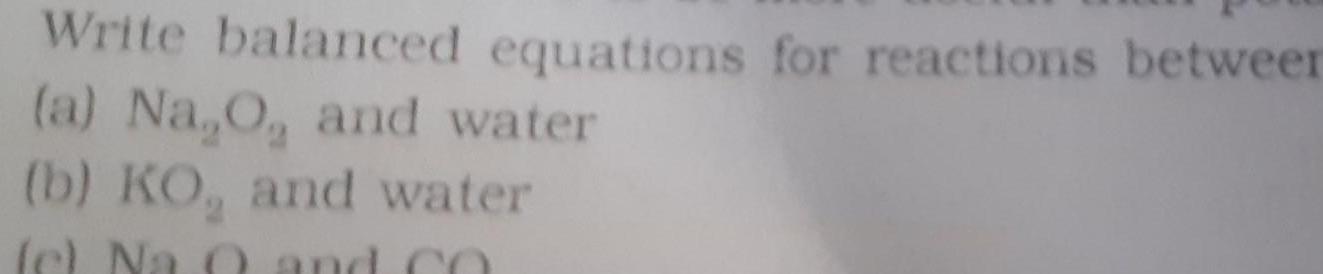
Physical Chemistry
GeneralWrite balanced equations for reactions between a Na O and water b KO and water c Na 0 and CO
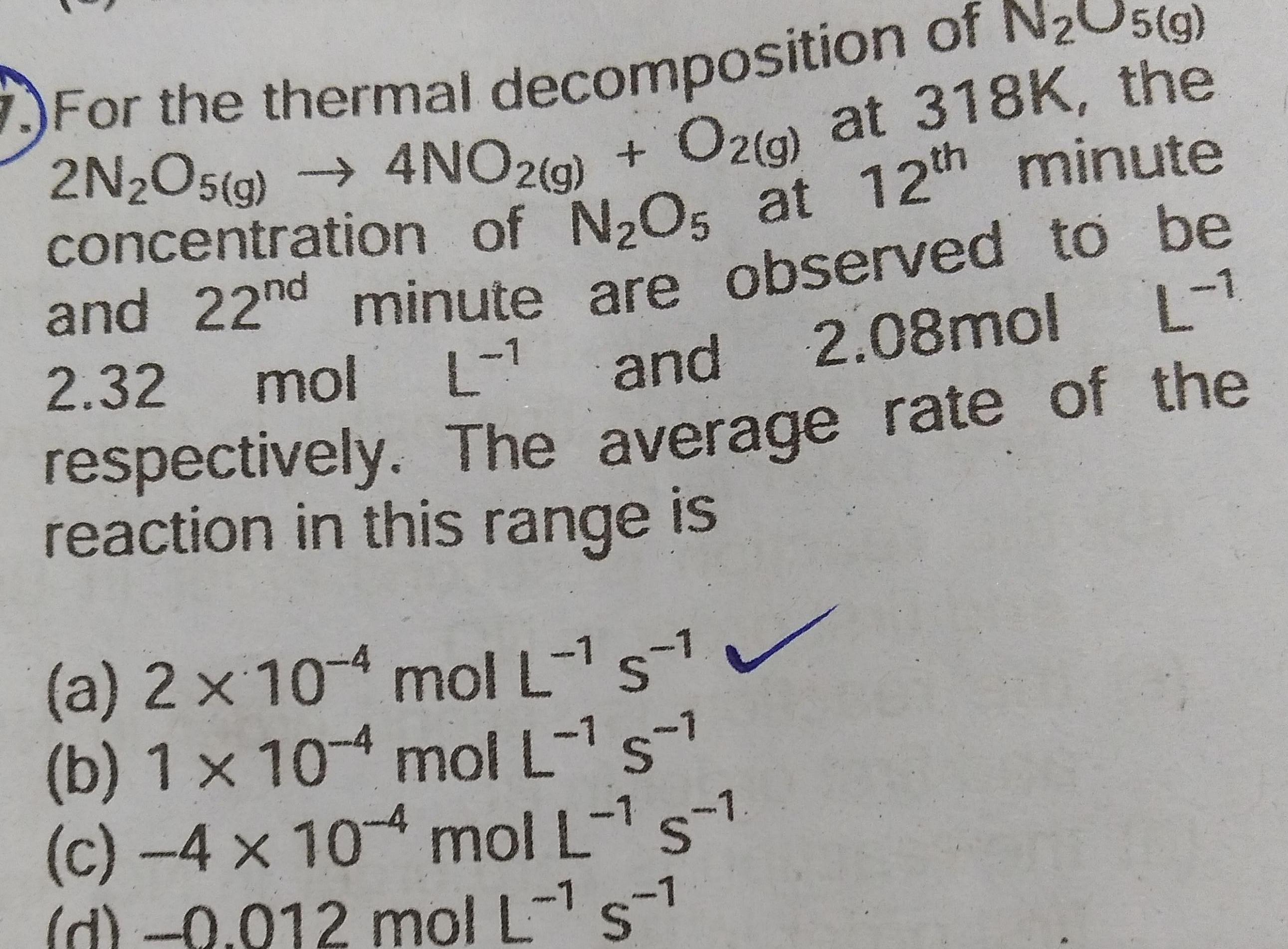
Physical Chemistry
Chemical kineticsFor the thermal decomposition of N O5 g 2N2O5 g 4NO2 g O2 g at 318K the concentration of N O5 at 12th minute and 22nd minute are observed to be 2 32 mol L and 2 08mol L respectively The average rate of the reaction in this range is a 2 x 10 4 mol L 51 b 1 x 104 mol L s c 4 x 10 4 mol L s 1 d 0 012 mol L s 1 1

Physical Chemistry
Gaseous and liquid statesFour particles have speed 2 3 4 and 5 cm s respectiv Their R M S speed is 1 3 5 cm s 3 54 cm s 2 27 2 cm s 4 54 4 cm s

Physical Chemistry
Gaseous and liquid states1 Triple point of water exists at 1 T 0 0098 C P 4 7 mm of Hg 2 T 0 98 C P 470 mm of Hg 3 T 25 C P 760 mm of Hg 4 T 298 C P 760 mm of Hg

Physical Chemistry
Gaseous and liquid statesThe work done which is to be required to increase the area of soap film of 10 cm x 6 cm to 10 cm x 11 cm is x 10 4 J The surface tension of the soap film is 2 3 x 10 3 Nm 1 4 3 x 10 1 Nm 1 1 3 x 10 4 Nm 1 3 3x 10 2 Nm 1

Physical Chemistry
Gaseous and liquid statesThree grams of helium diffuses from a container in 1 min The mass of sulphur dioxide diffusing from th same container over the same time interval will be 1 3 g 2 6 g

Physical Chemistry
Chemical kineticsExcluded volume of a gas will be larger if 2 Large 4 less than 1 1 Small 3 1 is

Physical Chemistry
Chemical BondingThe dissociation constants for acetic acid and HCN at 25 C are 1 5 x 10 5 and 4 5 x 10 10 respectively The equilibrium constant for the equilibrium CN CH3COOHHCN CH3COO would be A 3 0 x 105 B 3 0 X 10 5

Physical Chemistry
ElectrochemistryConsidering entropy S as a thermodynamic parameter the criterion fo any reaction to be spontaneous is O ASsystem ASsurroundings 0 O AS system 0 O AS surroundings 0 O ASsystem AS surroundings V 0
Physical Chemistry
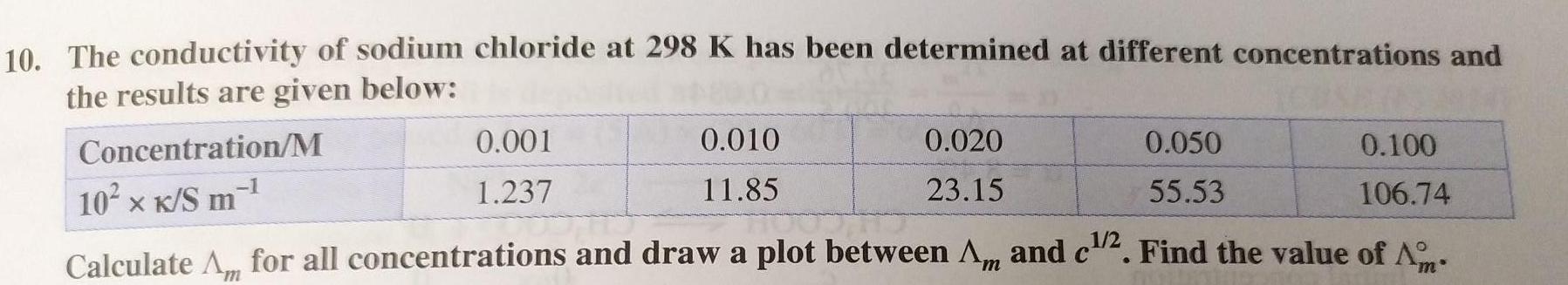
Electrochemistry10 The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below Concentration M 10 x K S m 0 001 1 237 1 0 010 11 85 0 050 0 100 55 53 106 74 1 2 Calculate Am for all concentrations and draw a plot between Am and c 2 Find the value of A C 0 020 23 15
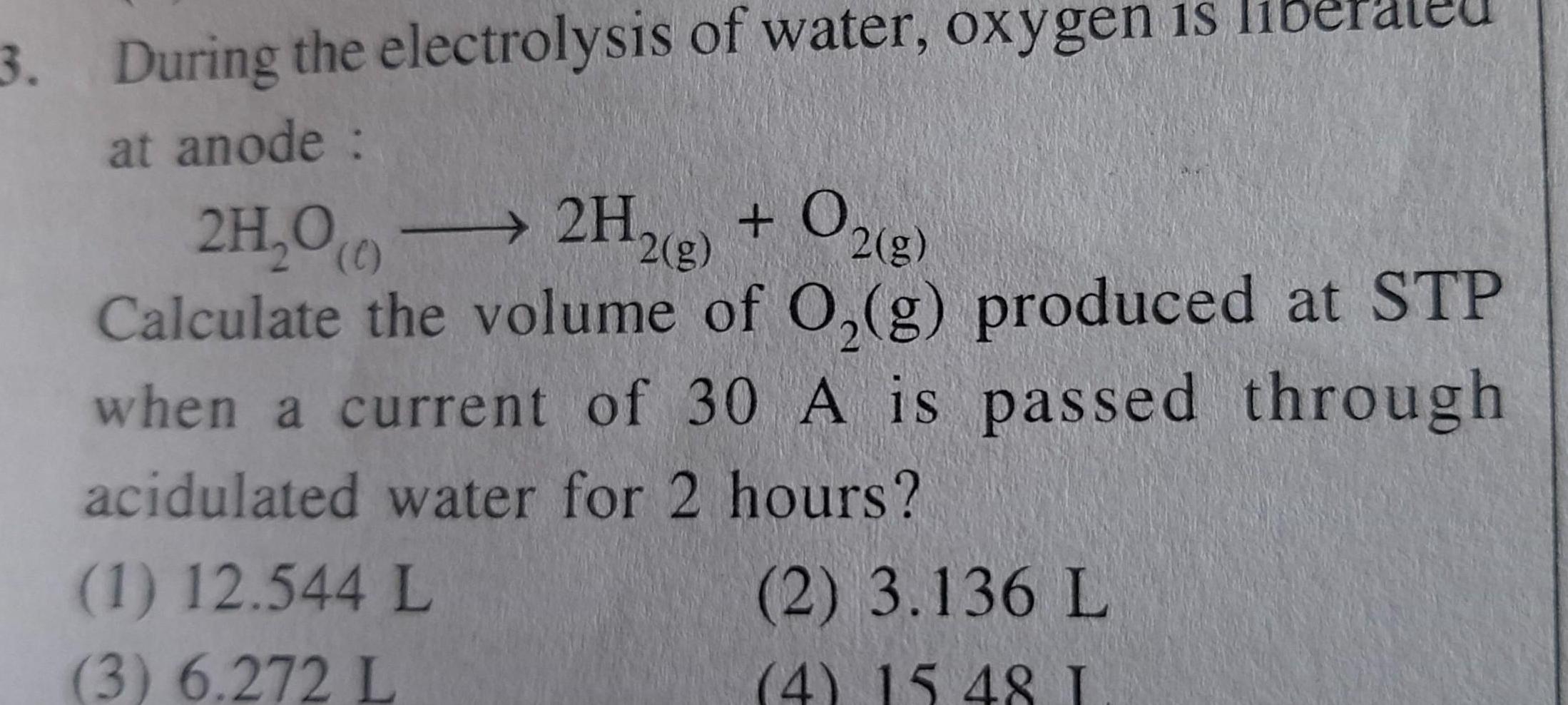
Physical Chemistry
Electrochemistry3 During the electrolysis of water oxygen is at anode 2H 00 2H2 g O2 g Calculate the volume of O g produced at STP when a current of 30 A is passed through acidulated water for 2 hours 1 12 544 L 3 6 272 L 2 3 136 L 4 15 48 I

Physical Chemistry
Gaseous and liquid statesA flask filled with CCl4 vapour was weighed at a temperature and pressure The flask was then filled with oxygen at the same temperature and pressure The mass of CCI vapour would be about 1 the same as that of the oxygen 2 one fifth as heavy as oxygen 3 five times as heavy as oxygen 4 twice as heavy as oxygen NIST

Physical Chemistry
Chemical kinetics2 Which of the following is are correct for the first order reaction log a x T112 Time I Initial Conc a III 1 I log a a x rate 2 11 Time 4 M II Initial Conc a IV

Physical Chemistry
Nuclear chemistryILLUSTRATION 9 Find out the total number of a and 90 B particles emitted in the disintegration of Th232 to 82Pb 208

Physical Chemistry
GeneralGiven that i 2C s 20 g 2CO g AH 786 4 kJ ii H g 1 2 O g H O l AH 285 8 kJ iii C H g O2 g 2CO g H O l AH 1297 kJ The enthalpy of formation of ethyne C H O 224 8 kJ O 224 8 kJ O 1072 2 kJ O 571 6 kJ

Physical Chemistry
Gaseous and liquid statesA gaseous mixture contains three gases A B and C with a total number of moles of 10 and total pressure of 10 atm The partial pressures of A and B are 3 atm and 1 atm respectively and if C has molecular weight of 2 Then the amount of C present in the mixture will be 1 8 g 3 3g 2 12 g 4 6 g

Physical Chemistry
Surface chemistryWhich of the following is not correct energy is an extensive a Gibbs property b Electrode potential or cell potential is an intensive property c Electrical work AG d If half reaction is multiplied by a numerical factor the corresponding E value is also multiplied by the

Physical Chemistry
Gaseous and liquid states5 Find the temperature at which the numerical values P of K and K will be equal to each other for the reaction N g H g NH3 g Ans In case of the given reaction An 1 or C numerical values of Kp and Kc be x then for the above reaction Kp x atm and K x mol L x L mol Therefore K K RT x atm 1 T 3 x L mol 1 x 1 If the 12 18 K The numerical values of K and K for the given reaction p C 1 0 0821 L atm mol K T

Physical Chemistry
SolutionsThe total number of electrons present in 18 mL of water 1 6 02 x 1022 2 6 02 x 1023 3 6 02 x 1024 4 6

Physical Chemistry
GeneralThe inversion of cane sugar proceeds with half life of 500 minutes at pH 5 for any concentration of sugar However if pH 6 the half life changes to 50 minutes The rate law expression for the sugar inversion can be written as 1 r k Sugar H 6 2 r k Sugar H 3 r K Sugar H 1 4 r K Sugar H

Physical Chemistry
Generalev 5 The energy of an electron moving in nth Bohr s orbit of an element is given by En n eV atom Z atomic number The graph of E vs Z2 keeping n constant will be Z E gros Z O w Z d E 13 6 72 Z

Physical Chemistry
Gaseous and liquid statesA bubble of air is present in underwater at temperature 15 C and the pressure 1 5 bar If the bubble rises to the surface where the temperature is 25 C and the pressure is 1 0 bar what will happen to the volume of the bubble 1 Volume will become greater by a factor of 2 5 2 Volume will become greater by a factor of 1 6 3 Volume will become greater by a factor of 1 1 4 Volume will become smaller by a factor of 0 70

Physical Chemistry
Chemical kinetics4 The conversion of A to B follows a second order kinetics Doubling the concentration of A will increase the rate of formation of B by a factor of c 4 a 1 2 b 2 ANGESPULEATPASION IN d 4

Physical Chemistry
Solid stateAB crystal has CsCl type structure If edge length of unit cell is 100 pm then nearest distance between cation and anion is mf w x 1 50 pm 3 1732 pm 2 100 pm 4 86 6 pm

Physical Chemistry
GeneralIn a gaseous reaction of the type aA bB cC dD which statement is wrong 1 a litre of A combines with b litre of B to giv C and D 2 a mole of A combines with b moles of B to giv C and D 3 g of A combines with b g of B to gi C and D 4 a molecules of A combines with b molecules B to giv Cand D R

Physical Chemistry
Chemical kineticsWhat is the relationship between coefficients of reactants in a balanced equation for an overall reaction and exponents in rate law In what case the coefficients are the exponents

Physical Chemistry
ElectrochemistryHalf of a substance is consumed in 40 minutes When the quantity of the substance is decreased to half the half life of the change is 20 minute The order of the reaction is 1 zero 2 1

Physical Chemistry
Chemical kineticsTwo first order reactions proceed at 25 C at the same rate The temperature co efficient of the rate of first reaction is 2 and that of the second reaction is 3 Find the ratio of rates of the reaction second and first reaction at 75 C 1 7 6 3 10 4 2 9 5 4 12 6

Physical Chemistry
ElectrochemistryReduction potential of half cell AgI aq saturated Ag E 10 16 is V 0 328 c 0 328 Agt Ag 0 8 V Ksp of AgI is b 1 272 0 146 3 1 fo