Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
ElectrochemistryFor the reaction Ni s Cu2 1M Ni29 1M Cu s E cell 0 57V AG of the reaction is a 110 kJ b 110 kJ c 55 kJ d 55 kJ

Physical Chemistry
Gaseous and liquid statesorder first B For the following reaction taking place at 400 K 2CO g CO2 g C s the total pressure is found to be 0 24 atm when the reaction is 100 complete Then the initial pressure of CO would have been

Physical Chemistry
Chemical BondingALLEN is a un CAREER INSTITUTE KOTA RAJASTHAN IV XeO 4 D I II III In which of the following species presence of double bond does NOT affect idealised bond angle re 1 POCI III XeO F C III IV only II XeO F B I II only fa

Physical Chemistry
GeneralSECTION I STRAIGHT OBJECTIVE TYPE P N and H are taken in 1 3 molar ratio in a closed vessel to attain the following equilibrium N g 3H g 2NH g Find k for reaction at total pressure of 2P if PN at equilibrium is A 3P 8 1 B 3p C 4p 3 D None

Physical Chemistry
ElectrochemistryThe emf of the cell 1 1 10 PL H HAHCIH Pt 0 0IM IM 2 1 10 is 0 295V Dissociation constant of the acid HA is x 0 1 10 s 3 1 10 8 n 03 Pra

Physical Chemistry
Chemical kineticsILLUSTRATION 11 Show that in a first order reaction time required for completion of 99 9 is 10 times of half life 2 NCERT of the reaction

Physical Chemistry
Solutions21 Ammonia gas is passed into water yielding a solution of density 0 93 g cm and containing 18 6 NH by weight The mass of NH3 per cc of the solution is 1 0 17 g cm 3 0 51 g cm 2 0 34 g cm 4 0 68 g cm

Physical Chemistry
Atomic StructureThe de Broglie wavelength asociated with particle of mass 10 kg moving with a velocity of 10ms is AIIMS b 6 63x10 16 d 6 63x10 29 a 6 63x10 7 c 6 63 10 21

Physical Chemistry
EquilibriumAt 400 C H g and I g are allowed to react in a closed vessel of 5 L capacity to produce HI g At equilibrium the mixture in the flask is found to consist of 0 6 mol H g 0 6 mol I g and 3 5 mol HI g Determine the value of K of the reaction C

Physical Chemistry
Chemical kineticsThe specific reaction rate of a first order reaction is 9 212 x 10 2 s If the reaction started with 2 mol L 1 calculate the amount of the reactant remaining after 50 seconds a 1 36 mol L 1 c 0 22 mol L b 0 632 mol L 1 d 0 02 mol L

Physical Chemistry
Solid stateIn ionic crystal AB if radius of cation and anion is 30 pm and 60 pm respectively then crystal structure is 1 ZnS type 3 CSCL type 2 NaCl type glt 4 B O type 12

Physical Chemistry
ElectrochemistryA drop of a solution volume 0 05 mL contains 6 x 10 7 mol of H If the rate of disappearance of H is 6 0 x 105 mol L 1 s 1 how long will it take for the H in the drop to disappear completely 1 8 0 x 10 8 S 3 6 0 x 10 6 S 2 2 0 x 10 8 s 4 2 0 x 10 2 S

Physical Chemistry
Chemical kineticsThe following graph shows how t 2 half life of a reactant R changes with the initial reactant concentration ao 1 2 1 a The order of the reaction will be 1 O 3 2 2 1 4 3

Physical Chemistry
General9 At 20 C 0 258 mol A g and 0 592 mol B g are mixed in a closed vessel of 5 L capacity to conduct the following reaction A g 2B g C g If 0 035 mol C g remains in the equilibrium mixture then determine the partial pressure of each constituent at equilibrium Ans According to the equation 1 mol A g reacts with 2 mol B g to produce 1 mol C g Hence 0 035 mol A g and 2 0 035 0 07 mol B g are required to produce 0 035 mol C g Therefore equilibrium molar concentrations of different constituents will be as follows No of moles at equilibrium A g 0 258 0 035 0 223 2B g 0 592 0 07 0 522 C g 0 035 0 223 Equilibrium conc mol L 0 0446 As given T 273 20 K 293 K At equilibrium PA A RT 0 0446 0 0821 293 1 072 atm PB B RT 0 1044 0 0821 293 2 511 atm Pc C RT 7 x 10 3 x 0 0821 293 0 168 0 522 5 0 1044 0 035 7 x 10 3

Physical Chemistry
EquilibriumD The information is insufficient to decide the In a basic aqueous solution chloromethane undergoes a substitution reaction in which CI is replaced by OH as CH Cl aq OH CH OH aq Cl aq The equilibrium constant of above reaction K 1 x 10 6 If a solution is prepared by mixing equal volumes of 0 1 M CH Cl and 0 2 M NaOH 100 dissociated then OH concentration at equilibrium in mixture will be A 0 1 M B 0 5 M C 0 2 M D 0 05 M

Physical Chemistry
GeneralOn diluting the solution of an electrolyte a both and k increase b both and k decrease c A increases and k decreases d A decreases and k increases

Physical Chemistry
ElectrochemistryTwo solutions concentrations conductivities have the ratio of their 0 4 and ratio of their 0 216 The ratio of their molar conductivities will be b 11 574 d 1 852 a 0 54 c 0 0864

Physical Chemistry
ElectrochemistryStrong electrolytes are given NaOH KOH NaCl CH COONa BaC A B C D Molar conductivity of Ba OH 2 at infinite dilution can be determined with the help of molar conductivity at infinite dilution of O A B and D OA C and D O A C and E OB C and D E

Physical Chemistry
Chemical kineticsThe temperature coefficient for the saponification of ethyl acetate by NaOH is 1 75 The activation energy is log 1 75 0 243 1 10 2 kcal mol 1 3 30 kcal mol 1 2 15 4 kcal mol 1 4 40 kcal mol 1

Physical Chemistry
Chemical kineticsFor a chemical reaction aA bB cC dD The ratic of rate of disappearance of A to that of appearance of C is 1 0 0 2 C a

Physical Chemistry
General67 Consider the reaction 2A B 3CP 20 Starting with 3 mole of A 2 mole of B and 6 mole of C number of moles of the products P and Q would respectively be a 2 and 4 b 4 and 2 c 3 and 1 5 d 1 5 and3

Physical Chemistry
Chemical kineticsILLUSTRATION 4 Identify the reaction order from the following rate constants NCERT 1 k 2 3 x 10 5 L mol 1 s 1 CA

Physical Chemistry
Chemical kineticsIf the rate of reaction is 2 6 10 3 mol L 1s 1 at 50 C and 7 02 10 2 mol L 1s 1 at 80 C then what will be the temperature coefficient of the reaction

Physical Chemistry
Chemical kineticsILLUSTRATION 9 A first order reaction is found to have a rate constant k 5 5 x 10 14 s 1 Find the half life of the reaction NCERT

Physical Chemistry
Chemical kineticsFor a reversible reaction of the type mAnB it was found that the concentration of A and B are the same at equilibrium k and k are the rate constants of the forward and backward reaction at a given temperature Which of the following relations is correct

Physical Chemistry
Chemical kineticsA three step reaction takes place such that net rate k k 3 k constant is k Ae 9 RT K Ae 10 RT K3 Ae 7 RT

Physical Chemistry
General27 Specific volume of cylindrical virus particle is 6 02 x 10 cc g whose radius and length are 7 and 10 respectively If N 6 02 x 1023 find molecular weight of virus a 15 4 kg mol b 1 54 x 10 kg mol c 3 08 x 10 kg mol d 3 08 x 10 kg mol 2001

Physical Chemistry
Gaseous and liquid statesAt 20 C 0 258 mol A g and 0 592 mol B g are mixed in a closed vessel of 5 L capacity to conduct the following reaction A g 2B g C g If 0 035 mol C g remains in the equilibrium mixture then determine the partial pressure of each constituent at equilibrium

Physical Chemistry
GeneralGive examples a Positive radicals b Basic radicals c Composite radicals d Metals with variable valenc e Bivalent acidic radicals f Trivalent basic radicals

Physical Chemistry
GeneralThe crystalline salt Na2SO4 xH O on heating losses 55 9 of its mass The formula of crystalline salt is A Na SO4 5H O C Na2SO4 3H O B Na SO4 7H O D Na SO4 10H O

Physical Chemistry
Chemical kineticsThe activation energy for the reaction 2HI H 1 is 184 kJ mol How many times greater is the rate constant for this reaction at 520 C than at 500 C R 8 31 JK 1 mol 1 1 0 5 3 5 5 2 0 18 4 2
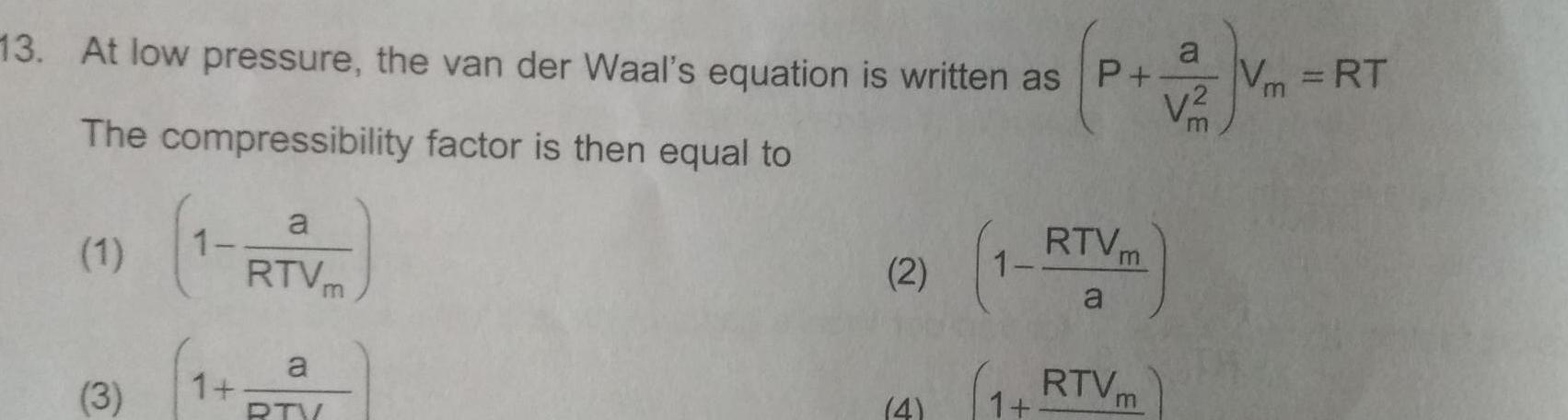
Physical Chemistry
Gaseous and liquid states13 At low pressure the van der Waal s equation is written as P V m The compressibility factor is then equal to 1 3 1 a RTV a RTV m 2 4 RTVm a 1 RTVm a V Vm RT

Physical Chemistry
Atomic StructureFor sodium atom number of electrons with m 0 will be 1 2 2 7 3 9 4 8

Physical Chemistry
Nuclear chemistryILLUSTRATION 5 Calulate the mass defect and binding energy per nucleon for 27C059 The mass of Co59 58 95 amu mass of hydrogen atom 1 008142 amu and mass of neutron 1 008982 amu

Physical Chemistry
Chemical kineticsQ7 The following data are for the decomposition of ammonium nitrite in aqueous solution Volume of N in c c 6 25 9 0 11 40 Time minutes 10 15 20 The order of reaction is 1 zero 3 two 13 65 35 05 25 2 one 4 three

Physical Chemistry
General5 The practice of looking down upon the Blacks is known as a religions c racial discrimination b racial law d imperialism

Physical Chemistry
Chemical kineticsFor a gaseous phase reaction 2A B 2AB the following rate data was obtained at 300K Rate of disappearance Concentration of B mol litre min i 1 8 10 3 ii 1 08 10 iii 5 4 10 3 The rate constant for the reaction is 1 0 5 mol 1 min 1 litre 2 0 8 mol min litre A 0 015 0 09 0 015 B 0 15 0 15 0 45

Physical Chemistry
Chemical kineticsThe decomposition of ozone proceeds as 1 03 0 0 fast ii 0 03 202 slow The rate expression should be 1 Rate K 031 2 2 3 Rate K 03 0 Rate K 03 0 022016 4 Rate K 03 0 1 1

Physical Chemistry
Gaseous and liquid states1 Air contains 23 oxygen and 77 nitrogen by weight The percentage of O by volume is 2 1 28 1 2 20 7 3 21 8 1

Physical Chemistry
Gaseous and liquid states5 Helium atom is two times heavier than a hydrogen molecule At 298 K the average kinetic energy of a Helium atom is 1 two times that of hydrogen molecule 2 same as that of a hydrogen molecule 3 four times that of a hydrogen molecule 4 half that of a hydrogen molecule

Physical Chemistry
Chemical kineticsThe rate of a certain reaction depends on concentration according to the equation K C 1 K C dC dt at very very high concentration what will be the order of the reaction

Physical Chemistry
GeneralIf 10 17 J of light energy is needed by the interio of human eye to see an object The photons o green light 2 550nm needed to see the objec are A 27 B 28 C 29 D 30
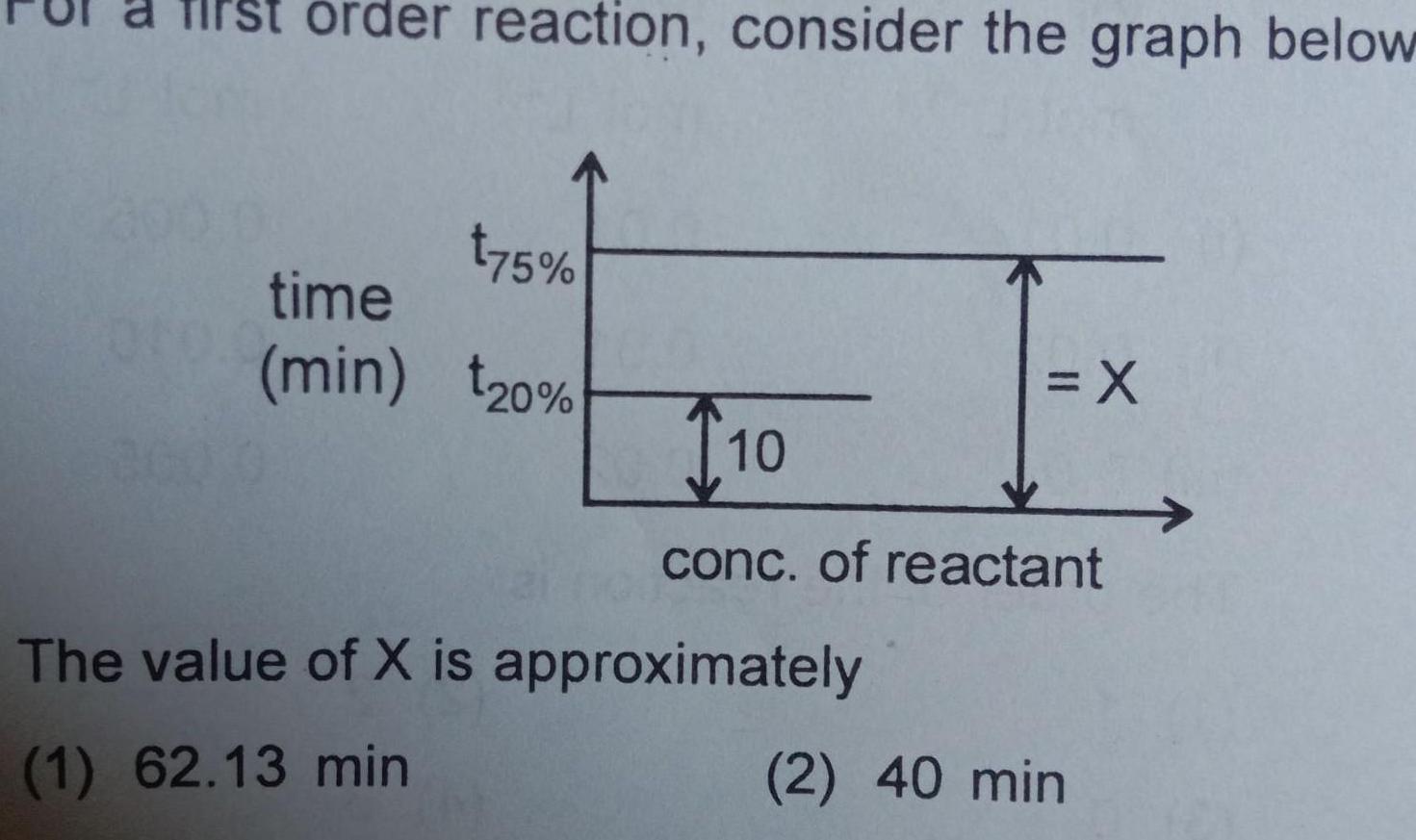
Physical Chemistry
Chemical kineticsorder reaction consider the graph below t75 time min 20 X T10 conc of reactant The value of X is approximately 1 62 13 min 2 40 min

Physical Chemistry
Solid stateCalcium metal crystallises in a face centered cubic lattice with edge length of 0 556 nm Calculate the density of the metal State Atomic mass of calcium 40 g mol N 6 022 x 1023 atoms mol 2 A

Physical Chemistry
Atomic StructureEqual moles of hydrogen and oxygen gases are placed in a container with a pin hole through which both can escape What fraction of the oxygen escapes in the time required for one half of the hydrogen to escape NEET 2016 11 12 2 1 2 NI 4 7 100 8 1 3 4 3

Physical Chemistry
GeneralThe oxidation reaction that takes place in lead storage battery during discharge is a Pb29 aq SO 2 aq b PbSO s 2H O 1 PbSO s PbO s 4H aq SO 20 aq 2e c Pb s SO2 aq d PbSO s 2e PbSO s 2e9 Pb s SO 20 aq

Physical Chemistry
ElectrochemistryBy diluting a weak electrolyte specific conductivity Kc and equivalent conductivity 2c change a A Both increase B Kc increases Ac decreases CKc decreases Ac increases D Both decrease

Physical Chemistry
General17 What would be percentage composition by volume of a mixture of CO and CH4 whose 10 5 mL requires 9 mL oxygen for complete combustion 1 CO 80 CH 20 3 CO 76 2 CH4 23 8 2 4 CO 90 CO 66 CH4 10 CHi 34

Physical Chemistry
Gaseous and liquid statesThe total number of valence electrons in 4 2 c N ion is NA is the Avogadro s number 2 4 2 NA 4 3 2 N 1 2 1 NA 3 1 6 N

Physical Chemistry
General4 In an experiment it showed that 10 mL of 0 05 M solution of chloride required 10 mL of 0 1 M solution of AgNO3 which of the following will be the formula of the chloride X stands for the symbol of the element other than chlorine a X Cl c XC14 b XCl d X Cl Karnataka NEFT 2013