Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
EquilibriumNa2CO3 aq 2HNO3 aq 2NaNO3 aq CO2 g H2O l If it required exactly 82 53 ml of HNO3 to completely react with 25 0 mL of 0 38 M Na2CO3 Determine the concentration in Molarity M of the HNO3 solution used in the reaction This is a titration problem


Physical Chemistry
EnergeticsThe rate of a certain reaction was studied at various temperatures The table shows temperature 7 and rate constant k data collected during the experiments Plot the data to answer the questions What is the value of the activation energy Ea for this reaction E kJ mol 1 What is the value of the pre exponential factor sometimes called the frequency factor A for this reaction A 8 1 T K 400 420 440 460 480 500 520 540 560 580 5 0 0000173 0 000127 0 000773 0 00403 0 0183 0 0738 0 267 0 879 2 66 7 43

Physical Chemistry
Atomic structureich sub shell corresponds to each of the following sets of quantum numbers a n 7 1 2 b n 3 1 0 c n 5 1 3 d n 2 1 1

Physical Chemistry
GeneralUsing either your lecture s textbook or an online resource search solubility curves of ionic compounds what is a possible identity of your unknown compound 378 18 23 16 9 989 Mass of unknown TOTAL MASS H O 9 7 00 mL 7 50 mL 8 00 mL 8 50 mL 9 00 ML 9 SOLUBILITY g solute 100 g H O 0 07 0 075 0 08 0 085 0 09 TEMPERATURE C 67 C 57 C 66 C 64 C 61 C

Physical Chemistry
EnergeticsFor the reaction NH4Cl aq NH3 g HCl aq AG 61 4 kJ and AH 86 4 kJ at 316 K and 1 atm This reaction is reactant product favored under standard conditions at 316 K The entropy change for the reaction of 1 54 moles of NH4Cl aq at this temperature would J K

Physical Chemistry
Solid stateFrenkel defect is just like interstiti al defect in ionic solids But ther e s a line in NCERT In Frenkel def ect density of solid doesn t chang e And In Interstitial defect dens ity of substance increase how th at s possible

Physical Chemistry
General78 10cm XPE Figure shows the vertical section of frictionless surface A block of mass 2 kg is released from the position A its KE as it reaches the position Cis A 40 14m Sm B 7m 1 180 J 2 140 J 3 40 J 4 2801 Abody constrained to move in the y direction is subjected 2x10x 114 v 2 X10X7 2 FT 3 7 The pot the x ax V x The total maximur 1 2 2 1 3 2 A body i


Physical Chemistry
General5 68 g of a mixture of CaCO3 and MgCO3 was dissolved in 800 ml of 0 4 M HCI and the solution was diluted to 1 lit 20 ml of this solution was neutralised by 20 ml of 0 1 M Na CO3 The percentage of MgCO3 in the mixture is approx 1 29 6 2 42 910

Physical Chemistry
General31 Ratio of C and C of a gas X is 1 4 The number of atoms of the gas X present in 11 2 litres of it at NTP will be a 6 02 1023 1989 c 3 01 1023 1 c c N O at NTP contains 1 8 a 1022 224 32 6 02 b Pov 22400 1 32 224 c atoms b 1 2 10231 d 2 01 1023 1023 molecules 1023 electrons 1988

Physical Chemistry
ElectrochemistryQ2 The resistance of a conductivity cell when filled with 0 02M KCI solution is 164 ohm at 298K However when filled with 0 05M AgNO3 solution its resistance is found it be 78 5 ohm If the specific conductivity of 0 02M KCI is 2 768 x10 ohm cm 1 Calculate A The specific conductivity L of 0 05M AgNO3 B The molar conductivity of AgNO3 Solution

Physical Chemistry
General10 The reaction A B follows first order kinetics The time taken for 0 8 mole of A to produce 0 mole of B is 1 hour What is the time taken for conversion of 0 9 mole of A to produce 0 675 m of B a c 0 25 hour 1 hour b 0 5 h d 2 hour

Physical Chemistry
GeneralGive the order of the densities from lowest to highest of the substances shown in the sketch Wood floats on water but sinks in oil oil wood water Suggest a procedure for measuring the density of a liquid Suggest a procedure for measuring the density of a solid that does not have
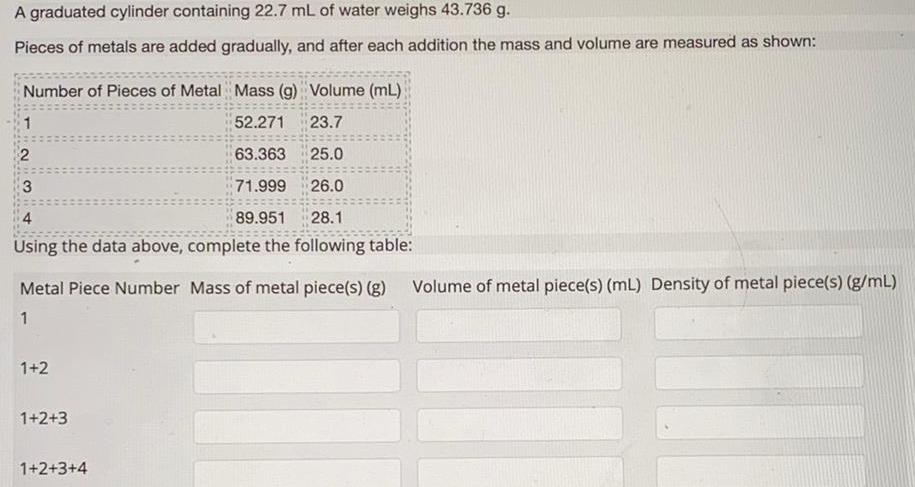
Physical Chemistry
GeneralA graduated cylinder containing 22 7 mL of water weighs 43 736 g Pieces of metals are added gradually and after each addition the mass and volume are measured as shown Number of Pieces of Metal Mass g Volume mL 52 271 23 7 63 363 25 0 3 71 999 26 0 4 89 951 28 1 Using the data above complete the following table 1 2 Metal Piece Number Mass of metal piece s g Volume of metal piece s mL Density of metal piece s g mL 1 1 2 1 2 3 1 2 3 4

Physical Chemistry
EnergeticsSECTION I A Write a note on physical signification of entropy Calculate change in entropy when 1 gm mole of two ideal gases mixed at 1 atmosphere isothermally R 1 987 cal mole degree Sit 7 7

Physical Chemistry
Equilibrium3 40 J 2x10x2 Abody constrained to move in the y direction is subjected to a force F 21 15j 6k newton The work done by this force in moving the body through a distance of 10 m along y axis is S 1 100 J 17100m 2 150 J 3 120 J 4 200 self propelled vehicle of mass m whose engine delivers V v 2 mv 15 1 2 mv 3 2 4 2 A body is moved ald delivering constant pow in time t is proportiona 1 1 2 2 3 4 3 AT1 Choose the correct plo for the following poten

Physical Chemistry
Equilibrium5 A 50 0 mL solution of 0 250 M lactic acid HC Hs0 Ka 1 4 x 104 is titrated with 0 400 M NaOH 16 What volume of NaOH solution is required to reach the equivalence point M J V2 som spont

Physical Chemistry
General1 0 g of magnesium is burnt with 0 56 g 0 in a closed vessel Which reactant is left in excess and how much 2014 At wt Mg 24 O 16 a Mg 0 16 g c Mg 0 44 g b 0 0 16 g d 0 0 28 g

Physical Chemistry
Equilibrium91 If A 8 2C 0 then angle between 24 and 2c will 91 aft A 36 20 02 2 1 60 2 120 1 60 3 30 2 120 4 150 3 30 A 4 150 92 If angle making by any vector with x axis a 22 and 92 fe fut fer un with y axis R 5000 30 2 6 9 1 3 2 ANBB 20 7 13 9 PCB 1

Physical Chemistry
Generalwith respect to time of flight from the shortest time of night to the longest y m 150 100 75 50 50 60 450 30 for the five path of the figure show 123 fa fa fa v 50 m s 15 100 150 200 Pasino ako T R 250 x m Lampo T sino la sino TAB 1 15 30 45 60 75 2 75 60 45 30 15 3 15 75 30 60 45 4 30 60 15 45 75 A particle is projected from the ground with an initial speed 124 of v at an angle e with horizontal un y m 150 100 75 50 Spartan Batch MEC KTG 2 D Relative Motion fa m after affer 60 459 30 58 m s 15 1 0 0 150 To a sino 1 15 30 45 60 75 2 75 60 45 30 15 3 159 75 30 60 45 4 30 60 15 45 75 200 Tasino Po 250 n sino sk x m Tasino RA 190 30

Physical Chemistry
Chemical kineticsMcQuarrie Rock Gallogly Consider the initial rate data at a certain temperature in the table for the reaction described by 2 NO g O g N O g O g Determine the value and units of the rate constant k k 2 99 X104 Incorrect NO2 o M 0 650 1 10 1 76 Units presented by Macmillan Learning 03 o M 0 800 0 800 1 40 M 5 1 Initial rate M s 2 99 104 5 06 x 104 14 17 x 104

Physical Chemistry
Chemical kinetics5 7 m sec mid way between the two directions 4 5 m sec at an angle off tan 4 3 with the directions of intial velocity v J VEIXY 4 The width of the 3 4 Raftan 4 3 5 m s 20m s at an ang VC 168 4 time to cross the 130 A man A is sitting in the rear end of a long compartment of 130 A crusta 1 2 s 2 a train running at constant horizontal velocity tosses a coin to a person B near the front end of the compartment The trajectory of the coin is as seen by B and a person C on the ground will have 1 different vertical ranges but equal horizontal ranges 2 equal horizontal and equal vertical ranges 3 equal vertical ranges but different horizontal ranges 4 different vertical and different horizontal ranges 1 Three ships A Band Care in motion The mati B THE 1 2 3 4 er en B 1 CR Fe TECHE BIGG DIH A 2 3 2 3 4 Data not su A particle is pr Cits horizontal other extremit the triangle


Physical Chemistry
GeneralFill in the chart about the following particles Charge Location in Atom Protons Neutrons Electrons Job What happens if you change the number of them in atom

Physical Chemistry
Atomic Structure3 Which one of the following arrangements represents the correct order of least negative to most negative electron gain enthalpy for C Ca Al F and O a Al Ca 0 C F b Al O C Ca F c C F O Al Ca d Ca Al C O F Karnataka NEET 2013


Physical Chemistry
Atomic structureChoose the one set of quantum numbers that contains an error On 3 1 2 ml 1 On 5 1 3 ml 2 On 3 1 2 ml 0 On 5 1 4 ml 3 On 3 1 0 ml 2

Physical Chemistry
EnergeticsDetermine the amount of heat in KJ required to heat 36 g of liquid Ca at 834 C to 989 C Melting Point 851 C Boiling Point 1487 C Molar Heat Capacities Csolid 26 2 J mol C Cliquid 31 0 J mol C AH fusion 9 33 kJ mol AHvaporization 162 kJ mol

Physical Chemistry
General3 1 10 versus time graph on the left The particle s position at t Os is x 10m v m s 10 10 0 5 0 5 x m 54 x m 10 5 4 2 0 2 The diagrams cho Versus time graph goes with the velocity 26 f 494 95 41 When a 2 kg car driven at Gurukripa furug at feafet 0sx 10m 2 5 5 10 10 SI t s t s 10 2 4 10 10 5 0 5 10 x m 10 5 0 5 t s x m 5 10 10 t s t s 1 x m 10 5 0 5 10 x m 10 5 of 5 10 4 2 0 2 v m s 5 5 5 10 t s 10 2 t s 10 4 t s x m 10 5 0 5 10 x m 10 5 0 5 10 5 10 denly put into neutral gear locity decreases in the foll IES VH where t is the time in se instant its speed is 10m 1 1 4 m s 2 1 2 m s 3 1 m s 4 3 4 m s A car is moving unif appears att 0 in fr the driver sees the The following is th stops at a distance t s 1

Physical Chemistry
GeneralScale reading Son who weights w newtons stands on a scale in an elevator that is initially at rest The elevator accelerates up ward to a constant speed and then slows to a stop Which of the following graphs best represents the reading on the scale He 1 Time Scale reading B Scale reading It 3 Scale reading Time Time Time 4 Werft var fore on f for fr Jie cafea shaft fase fieft want man a 2 Scale reading Scale reading Scale reading Scale reading Time Time Time Time and ti frafafena cenfant JE my W a 20 C

Physical Chemistry
GeneralThe rate constant for this first order reaction is 0 0158 s at 300 C A products If the initial mass of A is 18 19 g calculate the mass of A remaining after 1 90 min mass of A



Physical Chemistry
GeneralLeft A long spring is stretched by 2 cm its potential energy is U If the spring is stretched by 10 cm the potential energy stored in it will be 5 2am 4 1 y 10cm sy Figure shows the vertical section of frictionless surface A block of mass 2 kg is m 1 U 25 2 U 5 3 SU 4 25U

Physical Chemistry
Chemical BondingIn which of the following arrangements the given sequence is not strictly according to the property indicated against it a HF HCl HBr HI increasing acidic strength b HO HS H Se H Te increasing pK values 2 2 2 2 a c NH3 PH3 AsH3 SbH3 increasing acidic character d CO SiO2 SnO PbO increasing oxidising

Physical Chemistry
Chemical kineticsFor the two first order rea terms of temperature are given below AP K 1015 e BQ K 1014 e 1 217 C 3 500 C 1500 T 1000 T If initially t 0 both reaction started with same initial concentration of A and B then at what temperature both the reaction will have same initial rate NCERT Pg 112 2 217 K 4 500 K

Physical Chemistry
Chemical kineticsa 4 b 8 5 The rate of a first order reaction is 0 04 mol L s at 10 sec and 0 03 mol L s at 20 sec after initiation of the reaction The half life period of the reaction is a 34 1 s b 44 1 s c 54 1 s d 24 1 s

Physical Chemistry
Chemical kinetics10 of 36 Using the data in the table calculate the rate constant of this reaction k A BC D Trial A M 1 2 3 Units 0 360 0 360 0 432 B M 0 200 0 440 0 200 Rate 0 0 0 1 0 02

Physical Chemistry
Chemical kineticsion 24 of 36 Consider the reaction data What two points should be plotted to graphically determine the activation energy of this reaction To avoid rounding erre at least three significant figures in all values x1 A products T K k S 325 0 378 625 0 899 x2 y1 y2 Determine the rise run and slope of the line formed by these points

Physical Chemistry
Chemical kineticsQuestion 3 of 36 Determine the average rate of change of B from t 0 s to t 302 s A2B rate 0 00315 Incorrect M S Time s 0 151 302 Concentration of A M 0 740 0 450 0 160

Physical Chemistry
GeneralConsider the rate law rate k A Determine the value of x if the rate doubles when A is doubled x Determine the value of x if no change in rate occurs when A is doubled x

Physical Chemistry
EquilibriumA mixture of 100 m mol of Ca OH 2 and 2g of sodium sulphate was dissolved in water and the volume was made up to 100 mL The mass of calcium sulphate formed and the concentration of OH in resulting solution respectively are Molar mass of Ca OH 2 Na2SO4 and CaSO4 are 74 143 and 136 g mol respectively Ksp of Ca OH 2 is 5 5 x 10 6 A 13 6g 0 28 mol L 1 B 13 6g 0 14 mol L e 1 9g 0 28 mol L D 1 9g 0 14 mol L 1

Physical Chemistry
GeneralThe half life period of a second order reaction is 30 minutes If the initial concentration is 0 1M the rate constant will be 1 0 666 M x min 2 0 333M x min 3 0 444M x min 4 0 555M x min

Physical Chemistry
EquilibriumFor the reaction 2NO g O g 2NO g the volume is suddenly reduced to half of its value by increasing pressure on it If reaction is of first order w r t O and second order w r t NO the rate of the reaction will NCERT Pg 106 1 Decrease to 8 times of the initial value 2 Increase to 8 times of the initial value 3 Increase to 4 times of the initial value 4 Decrease to 4 times of the initial value

Physical Chemistry
EnergeticsMEC KTG 2 D Relative Mat 127 Two tall buildings are 30 m apart The speed with which a 127 3 m 30 m fra 150 m furgant van fer fee if an 2 RR 27 5 m forga ball must be thrown horizontally from a window 150 m above the ground in one building so that it enters a window 27 5 m from the ground in the other building is 1 2 ms 2 6 ms 1 3 4 ms 4 8 ms falling vertically The speed of rain w 1 0 5 m s 128 Two stones are projected with the same speed but making 128 TRT I F 2 0 52 different angles with the horizontal Their ranges are equal If the angle of project 1 2 ms 2 6 ms 3 4 ms 48 ms Iro 10 30m 9 7 5 150 Class X Batectronics Modern Phys 97 5 1995 Gurukripa 32 To a stationary man at an angle 30 we forward with speed

Physical Chemistry
Chemical BondingFor the following reaction which can be inferred from the figur A B conc A A B 50 Time min d A d B dt at t 0 dt B At least one intermediate is formed C The rate of formation of B is maximum at t 0 D the reaction reaches equilibrium in 50 minutes

Physical Chemistry
ElectrochemistryOver voltage is result of O O O O Discharge of H ions at the electrode surface 1 point Neutralization of the charge of the ions or protons by electrons Combination of H atom to form H2 molecules O All of the above

Physical Chemistry
General1 The formation of the oxide ion 02 from oxygen atom requires first an exothermic and then an endothermic step as shown below 1 O g e Qg A H 141 kJ mol Piste Oge0 A H 780 kJ mol 8 Thus process of formation of O in gas phase is unfavourable even though O is isoelectronic with neon It is due to the fact that a Orion has comparatively smaller size than oxygen atom b oxygen is more electronegative c addition of electron in oxygen results in larger size of the ion d electron repulsion outweighs the stability gained

Physical Chemistry
Chemical kineticsQuestion 9 of 36 Using the data in the table determine the rate constant of the reaction and select the appropriate units A 2BC D k Trial A M B M Rate M s 1 0 300 0 600 0 300 2 3 Units 0 220 0 220 0 440 0 0162 0 0162 0 0648