Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Gaseous and liquid statesThe densities of solid and liquid silver MW 107 9 g mol are 11 7 g mL and 10 5 g ml respectively The normal melting point of silver is 962 C The enthalpy of fus of silver is 12 0 kJ mol What is the melting point in C of silver under an applied pressure of 500 atm

Physical Chemistry
GeneralSuppose you have 600 0 grams of room temperature water 20 0 degrees Celsius in a thermos You drop 90 0 grams of ice at 0 00 degrees Celsius into the thermos and shut the lid a What is the equilibrium temperature of the system Provide your final answer with three significant digits of precision here b How much ice is left in grams

Physical Chemistry
Chemical kineticscalculate the packing fraction of BCC unit cell Half life of a first order reaction at a given temperature is a 3 min Calculate the time required for the completion of 3 4 th of the reaction a 12 min b 6 min c 24 min d 20 min mothoxymothon c HI

Physical Chemistry
GeneralA 1 000 g sample of an alum is dissolved and sulfate is precipitated as BaSO The precipitate weighed 0 466 g after it was washed dried ware in the form K Al SO 24H O calculate the percentage if ALO in the sample Given Atomic mass of Ba 137 S 32 Al 27 alum A 3 4 B 13 2 C 1 7 D 6 8

Physical Chemistry
GeneralAn experiment calls for 185 mL of a 1 55 M solution of hydrochloric acid Stock solutions of concentrated hydrochloric acid are 12 4 M How many mL of stock solution would need to be diluted in order to make the solution required for the experiment O 14 8 mL O 23 1 mL O 2 31 x 102 mL O 9 59 x 10 3 mL O 1 48 mL

Physical Chemistry
Chemical BondingElement A with minimum atomic number in which last electron has n m 3 but divisible by 3 bra How many given statements are correct about A n Principal quantum number m magnetic quantum number 1 Sum of group number and period number in Modern Periodic Table is even number ii Element belongs to s block iii Element belongs to IIIA group number iv Element is a metal v Atomic number of element is divisible by 3 vi Another element B has an atomic number atomic number of A 10 then element B belongs to III B group vii Element A has largest radius in their period viii The group in which element A is present has maximum elements in Modern Periodic Table to pa

Physical Chemistry
Atomic Structure3 pts For a pair of certain electron donor and acceptor pair A G 1 065x10 J Which scenario would you predict would have the fastest electron transfer A Distance is 20 angstroms reorganization energy is 5 065x10 19 J B Distance is 20 angstroms reorganization energy is 1 065x10 19 J C Distance is 60 angstroms reorganization energy is 5 065x10 19 J D Distance is 60 angstroms reorganization energy is 1 065x10 19 J 1

Physical Chemistry
Energetics8 Calculate the total heat in kJ required to convert 10 0 grams of liquid ethanol MM 46 07 g mol at its boiling point to gaseous ethanol at 158 C Note that not all of the data below is necessary for this problem AH 40 5 kJ mol AH 5 10 kJ mol 1 14 kl 6 28 kJ 9 93 kJ MP 115 C BP 78 C Cand 1 88 J g C Chand 2 45 J g C C 1 43 J g C

Physical Chemistry
GeneralCI None of these acid halide anhydride least reactive acid halide ester amide anhydride most reactive least reactive amide anhydride acid halide ester most reactive least reactive acid halide anhydride ester amide most reactive least reactive amide ester anhydride acid halide most reactive least reactive ester amide anhydride acid halide most reactive

Physical Chemistry
Chemical BondingXe Oa Calculate Bond Pairs BP s 1 mark Calculate Lone Pairs LP s 1 mark Draw Lewis Structure 1 mark VSEPR Notation 0 5 mark Predict of then molecular Shape 0 5 mark Polarity 0 5 mark Provide the reason and justify your answer for the polarity

Physical Chemistry
EquilibriumWhich of the compounds would have the weakest conjugate base O cyclohexanone with a Ka 5 X 10 16 O cyclopentadiene with a Ka 1 X 10 15 acetylene with a K 1 X 10 25 2 chloroethanol with a K 2 X 10 14

Physical Chemistry
Solutions3 5 Concentration of glucose C6H 2O6 in normal blood is approximately 90 mg per 100 mL What is the molarity of the glucose solution in blood 1 5 M 2 0 005 M 3 0 05 M 4 1 M

Physical Chemistry
General35 Match the metal Column I with its reaction with oxygen Column II A B a Column I Potassium b x c i ii iii iv A iv B iii C ii D i A iv B ii C i D iii A iii B ii C i D iv A iv B ii C iii D i C D Silver Zinc Copper Column II Does not react event at high temperatures Gets coated with black coloured layer of oxi Does not burn at ordinary temperature Burns vigorously

Physical Chemistry
Gaseous and liquid states1 12 pts Describe how you would measure the density at room temperature of a pure substance composed only of this molecule You will need to figure out if this substance is a solid liquid or gas at room temperature and make your plan according to this Be creative and assume you have access to whatever glassware equipment and instruments you need a Describe your stepwise procedure here Include details about the kind of glassware and equipment you are using for each step Make sure you include any calculations you would need to perform on your measurement s to determine the density b Choose one measurement e g mass volume length etc you described in a Describe one way

Physical Chemistry
Gaseous and liquid states1 A steel tank contains air at a pressure of 15 bar at 20 C The tank is provided with safty valve which can withstand a pressure of 35 bar Calculate the temperature to which tank can be safely heated

Physical Chemistry
SolutionsA Molarity Imole in slit Sol MATB 1 Molar V L 20 166 X 480 Imole in 980 Solut mole of solut volunce u seln m1 Soluti 50 wen 20gm y 1 45 M AB C MATA x 1000 X1000 molal Imole in sing of solvent v y 2 IND I more concentrated 1 202 Imole in Ike solut

Physical Chemistry
Generala For pure oxygen O2 gas at 0 C calculate the molecular density in the form of molecule cm3 in the following cases i and ii b In the following cases i and ii what is the average velocity of the N2 molecule 1 mole of oxygen atoms 16 g i at standard atmospheric pressure 1013 mbar normal pressure is accepted ii at 10 10 mbar pressure UHV and 1000 km above the ground surface Assume O2 gas behaves as an ideal gas

Physical Chemistry
EquilibriumSuppose a 250 mL flask is filled with 0 70 mol of H and 0 80 mol of HI This reaction becomes possible H g 1 g 2HI g 1 Complete the table below so that it lists the initial molarity of each compound the change in molarity of each compound due to the reaction and the equilibrium molarity of each compound after the reaction has come to equilibrium Use x to stand for the unknown change in the molarity of H You can leave out the M symbol for molarity initial change 0 X 1 0 HI 0 A X

Physical Chemistry
SolutionsA mixture of 20 ml of methane and 20 ml of O is exploded and cooled at room temperature If the reaction between the two substance is written as CH4 20 CO H O then final volume of the gaseous mixture is 1 10 ml 2 20 ml 3 30 ml 4 60 ml

Physical Chemistry
Solid stateWhich is the incorrect statement 2017 1 Density decreases in case of crystals with Schottky s defect se 2 NaCl s is insulator silicon is semiconductor silver is conductor quartz is piezo electric crystal 3 Frenkel defect is favoured in those ionic compounds in which sizes of cation and anions are almost equal 4 FeO0 98 has non stoichiometric metal deficiency

Physical Chemistry
EnergeticsAn ideal gas undergoes a mechanically reversible process constant pressure constant temperature and adiabatic process The gas entering a T1 650K and P1 10bar decreases its temperature at constant pressure where V2 2 91x10 3 m3 Then it went to isothermal process to decrease its pressure Finally the gas returns to its initial state Take Cp 7 2 and Cv 5 2 Calculate the following a T2 P3 V1 and V3 b Q W AU and AH

Physical Chemistry
Atomic Structures For a pair of certain electron donor and acceptor pair A G 1 065x10 19 J Which scenario would you predict would have the fastest electron transfer A Distance is 20 angstroms reorganization energy is 5 065x10 19 J B Distance is 20 angstroms reorganization energy is 1 065x10 9 J C Distance is 60 angstroms reorganization energy is 5 065x10 19 J D Distance is 60 angstroms reorganization energy is 1 065x10 19 1

Physical Chemistry
GeneralAn iron tank contains helium at a pressure Example 5 6 of 2 5 atmosphere at 25 C The tank can withstand a maximum pressure of 10 atmosphere The building in which tank has been placed catches fire Predict whether the tank will blow up first or melt The melting point of iron 1535 C

Physical Chemistry
Solid stateA NIST reference material containing 267mgL Fe was analyzed by spectrophotometric and titrimetric techniques giving the results in the table below Analytical technique Titrimetry Number Mean of replicates i 8 Spectrophotometry 10 mgL 249 283 Standard deviation mgL Fe 13 27 Determine whether the techniques can accurately quantify the analyte at the 95 confidence level Explain your reasoning ii Is there a significant difference between the mean and precision of the methods 5 12

Physical Chemistry
Chemical kineticsData for the dimerization 2A A of a certain nitrile oxide compound A in ethanol solution at 40 C follow A mmol dm 68 0 50 2 40 3 33 1 28 4 22 3 18 7 t min 0 40 80 120 160 240 300 Find the reaction order using the half life method 14 5 420
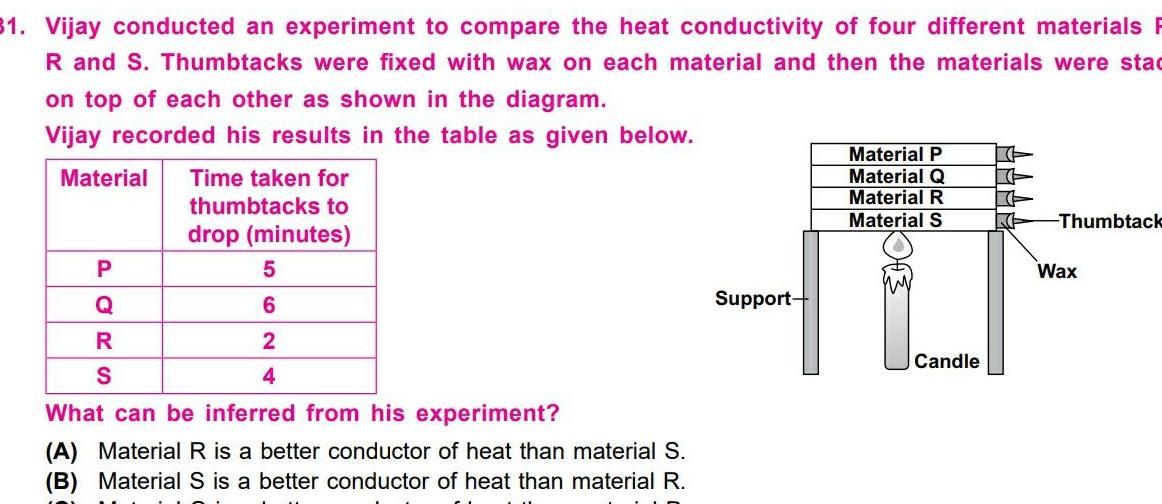
Physical Chemistry
General31 Vijay conducted an experiment to compare the heat conductivity of four different materials R and S Thumbtacks were fixed with wax on each material and then the materials were stad on top of each other as shown in the diagram Vijay recorded his results in the table as given below Material P Q R S Time taken for thumbtacks to drop minutes 5 6 2 4 What can be inferred from his experiment A Material R is a better conductor of heat than material S B Material S is a better conductor of heat than material R Support Material P Material Q Material R Material S Candle K Thumbtack Wax

Physical Chemistry
Solutions500 mL of 61 W V urea solution is mixed with 500 mL of 18 w V gluc ose solution and final volume of mixtur e becomes 2 L after addition of water The osmotic pressure of final 2 L soluti on at 27 degrees C is 5yR The value o fy is R universr gas constant

Physical Chemistry
EnergeticsHERE IN THE QUESTION WHY CANT WE DI RECTLY USE FORMULA WHICH HAS DELTA PV AND WE KNOW DELTA V IS O SO TERM WOULD BE 0 BUT INSTEAD WHY WE USE DELTA N GAS RT FORMULA I AM VERY C ONFUSED WHEN TO USED FORMULA WHIC H HAS DELTA PV AND WHEN TO USE DELT AN GAS RT Enthalpy of combustion For the complete combustion of ethanol C H OH 30 g 200 g 34 0 the amount of heat released as measured in a bomb calorimeter is 1 364 47 kJ mot at 300 K Assuming ideality what will be the enthalpy of combustion 4 H for the reaction 25 0 a 1 366 97 kJ mot b 1 361 95 kJ moft c 1 460 50 kJ mo d 1 350 50 kJ mo

Physical Chemistry
EnergeticsA group of students conducted an experiment using a coffee cup calorimeter Initially the calorimeter contained only deionized water and the temperature of the water was 21 24 C After adding a soluble ionic compound and stirring the temperature reached 23 56 C The temperature of the mixture rose because the water absorbed thermal energy from the ionic compound the solution absorbed heat from the surroundings the dissolution process was endothermic the dissolution process was exothermic

Physical Chemistry
Chemical Bonding1 What are the electron counts for the two complexes 2 Sketch the SALCs for the orbitals on the two ethylene ligands in the left complex and assign their Mulliken symbols in D2h point group symmetry H C CH Ph Ph Ph Ph H C CH B C6F5 3 C H6 H C Ph Mo Ph B C6F5 3 Ph Ph

Physical Chemistry
Chemical kineticsA 2 97L sample of gaseous HI having density of 12 619 g L is heated to 641 6F As time passes the HI decomposes to gaseous H and 12 The rate law at thi temperature is given by k 0 031 M min What is the partial pressure of H after a reaction time of 6 01h Record your answer in atm with 3 decimals

Physical Chemistry
Solutions12 10 gm of a sample of CaCO is treated with 200 ml of 0 1 N HCI Calculate the purity of CaCO 1 50 2 10 3 65 4 90 13 The number of moles of potassium permagnet required to oxidise 2 moles of oxalic acid in acidic medium is 1 3 1 6 11 2 is 4 14 The number of moles of ferrous oxalate oxidised completely by 1 mole of K Cr O in acidic medium 3 2 4 2 7 2 12 4 7 5 2 3 2 ANSWER 3 8 2 3

Physical Chemistry
General4 Use the Universal Soil Loss Equation USLE and the tables and graphs of values for USLE variables in Chapter 17 of Brady Weil to a determine if a 150 meter long tilled row crops field of Mexico silt loam located in McCredie Missouri on a 4 slope is classified as highly erodible land HEL The definition of highly erodible land is given in the footnote at the bottom of page 874 15th ed Assume the T value for this soil is 11 Mg ha yr

Physical Chemistry
Gaseous and liquid states5 1 g of NH3 and 3 g of NO are mixed and kept in a container of 1 L at 27 C The total pressure exerted by gases is x Then the value of 10 x in atm is Assume gases are non reacting and ideal Take R 0 08 atm L K mol Answer HII 0 1 2 3 4 5 6 7 8 9

Physical Chemistry
EnergeticsAn analytical chemist is titrating 242 1 mL of a 0 5100M solution of aniline C6H5NH with a 0 3200M solution of HIO3 The p K of anilin is 9 37 Calculate the pH of the base solution after the chemist has added 442 2 mL of the HIO3 solution to it Note for advanced students you may assume the final volume equals the initial volume of the solution plus the volume of HIO3 solution added Round your answer to 2 decimal places pH 0 X S

Physical Chemistry
GeneralCalculate the relative population of the J 1 rotational level of the ground vibrational state of HCl at 300 K Use the values Planck s constant h 6 626x10 Js velocity of light c 2 998 10 ms constant for the diatomic molecule B 10 4398 cm and k 1 3806 10 JK A 2 71 B 0 C 1 2 71 D 1 Answer OA OB OC OD questio Submit

Physical Chemistry
GeneralA2 3 7 5w Two substance A B are allowed to react completely to from A B A B mixture of leaving 1 4 none of the reactants Using this information calculate the composition of final mixture when mentioned amount of A B are taken 1 6 2 4 1 3 2 32 5 If moles of A 2 moles of B is taken 4 A A B 0 2 mole A B 0 3 mole A B 0 5 mole A B 0 5 mole If 7 moles of A 6 moles of B is taken A A B 1 mole A B 2 C A B 2 mole A B 4 If 4 moles of A 6 moles of B is taken A A B 2 mole A B 3 C A3B 4 mole A B 1 1 5 32 32 5x B A B4 0 8 mole A B 0 7 mole D A3B4 0 4 mole A B 0 6 mole z B A B 2 mole A B 1 D A B 4 mole A B4 1 x x S 2 B A B 2 mole A B 1 D A3B4 3 mole A B 2 7 2 X 1 07 21x4

Physical Chemistry
Surface chemistry10 g of gelatin is required to be added to 10 cm of a standard gold sol to just prevent its coagulation by the addition of 1 cm of 10 NaCl solution to it Hence the gold number of gelatin is 1 10 2 1 0 3 0 1 4 0 01

Physical Chemistry
EquilibriumAssertion A A Sodium hydrogen carbonate is used in fire extinguisher Reason R Sodium hydrogen carbonate is a weak base A I think the answer is b both assertion and r eason are correct and reason is not the corr ect explanation of assertion But answer given is a both are correct and r eason is correct explanation of assertion Please give your opinion and explain the an

Physical Chemistry
GeneralWhat is the change in internal energy when a gas contracts from 377 mL to 177 mL under a constant pressure of 1520 tort while at the same time being cooled by removing 124 J heat 40 52 J O 83 48J O 248J None of these

Physical Chemistry
GeneralA solid substance A decomposes into two gaseous product B and C as A 2B g C g If at equilibrium some C at 1 atm is added in constant volume condition 10 of Bg solidified before the equilibrium was re established What is the total pressure at final equilibrium

Physical Chemistry
Generalb Given that the value of Kc is 5 2 x 10 at 8592 K for which of the following concentrations of molecular gas would the small x approximation be valid x is 5 of the initial concentration or less 1 mark Select all that apply noting there is a penalty for incorrectly selected responces 0 01 M 0 03 M 0 1 M 0 3 M 1 M U 3 M 10 M None of these concentrations are suitable c At this temperature what is the concentration of oxygen atoms at equilibrium in a sealed chamber initially containing only

Physical Chemistry
GeneralHERE IT IS GIVEN THAT THE REACTION MU ST BE AT CONSTANT PRESSURE SO THE C ONTAINER MUST BE OPEN BUT IT ISNT O PEN HERE asurements at constant pressure For the measurement of heat transferred at constant pressure the calorimeter used should constitute an open system So any vessel kept open can be used along with a thermometer for the measurement of the temperature change and a stirrer for distributing the heat released throughout the solution The apparatus is shown in Fig 3

Physical Chemistry
General12 a Draw the kinetic and thermodynamic enolates of the ketone below 4 points A Kinetic enolate B Thermodynamic enolate C b On the reaction coordinate diagram identify which enolate fits in each empty box E IN

Physical Chemistry
GeneralThe magnetite is a mixed oxide of inon It consists of Feo and Fe 03 Assume that given sample was lying in contact with oxidising agent it contains more Fie 03 than Feo Given sample of magnetite on reaction with carbon monoxide forom iron metal and co If 39 2g of given sample of magnetite on reaction with sufficient carbon monoxide produces 15 68 L of CO gas at S T P Then 3 9 2g of given sample That is given in paragraph is completely oxidised into Fe by KMNO4 and K G 07 in different experiment x ml of 0 1 M KMNO4 Cin acidic medium is required for Complete oxidation of given sample while y ml of 0 1 M K C 07 is required for Complete oxidation Cin acidic medium select the incorrect statement a x 200 b y 166 67 771

Physical Chemistry
General2 Example 7 9 1 0 mol of H 2 0 mol of 1 and 3 0 mol of HI are injected in a 1 litre flask What will be the concentration of H I2 and HI at equilibrium at 490 C The equilibrium constant for the reaction at 490 C is 45 9

Physical Chemistry
Nuclear chemistryDetermine the half life of the substance that decays from A to A in time t A51 A 19 t 20 hours The half life of the substance that decays from 51 to 19 in 20 hours is hours Do not round until the final answer Then round to one decimal place as needed

Physical Chemistry
EquilibriumExample 7 7 Calculate the degree of ionization of pyridine CHN in its 0 1 M solution K for pyridine is 1 5 x 10 What would be the degree of ionization of pyridine if the solution is also 0 1 M in NaOH

Physical Chemistry
Nuclear chemistryMass defect in the nuclear reactions may be expressed in terms of the atomic masses of the parent and daughter nuclides in place of their nuclear masses 10 The mass defect of nuclear reaction Be 0 B 0 e is 5 A Am At mass of Be 0 At mass of B 0 10 10 of B 0 mass of one electron B Am At mass of Be 0 At mass C Am At mass of Be 0 At mass of B 0 mass of one electron 10 10 m At mass of Belo At mass of B 0 mass of two electrons 10 10 RA0064

Physical Chemistry
EquilibriumFind the pKa of the salicylic acid based on the pH obtained from the experiment below pH 6 15 10 ml of 0 5 w v solution of salicylic acid is used 0 5 N sodium hydroxide solution is used Results Volume of sodium hydroxide ml x Complete neutralization 0 6mL Volume of sodium hydroxide ml x 2 half neutralization 0 25 C 25 pH 6 15