Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
GeneralIn a serine protease cysteine can be substituted for serine and still retain nearly full catalytic activity What property of cysteine permits this A B C D When deprotonated the thiol group is a good nucleophile like the hydroxyl group The sulfur of cysteine is nearly the same size as the oxygen in serine Preferential binding of the transition state is the primary driver of the reaction Both A and C

Physical Chemistry
General9 24 Describe how to prepare 250 mL of 1 00 M ammonia buffer pH 9 00 starting with 28 wt NH3 concentrated ammonium hydroxide listed on the page after the periodic table and concentrated HC1 37 2 wt or concentrated NaOH 50 5 wt

Physical Chemistry
Atomic StructureFollowing are the advantages of double beam instrumentation EXCEPT A Compensate for all but most short term fluctuations in the radiant output of the sou as well as for drift in the transducer and amplifier B Suited for quantitative absorption measurements at a single wavelength C Compensate for wide variations in source intensity with A D Continuous recording of transmittance or absorbance spectra

Physical Chemistry
GeneralThe order of energy required to 1 point remove electron is as follows in mass spectrometry is o electrons non conjugated O conjugated non bonding or lone pair of electrons non conjugated conjugated n non bonding or lone pair of electrons o electrons conjugated non conjugated non bonding or lone pair of electrons o electrons non conjugated o electrons conjugated non bonding or lone pair of electrons

Physical Chemistry
GeneralAqueo Will react with aqueous magnesium chloride to give solid silver chloride and aqueous magnesium nitrate If 97 3 g of silver nitrate is allowed to react with 52 0 g of magnesium chloride and 72 4 g of the desired silver chloride is produced what is the percent yield of the reaction Your answer is understood to be a percentage please only answer numerically

Physical Chemistry
Chemical kineticsde Broglie wavelength in metre of a microscopic particle of 2 mg mass moving with speed of 3 3 m s will be Planck s constant h 6 6 10 34 Js Only One Correct Answer A 10 26 8 10 29 C

Physical Chemistry
GeneralChoose the incorrect 1 Above curie temperature ferromagnetic solid becomes paramagnetic 2 On heating ferrimagnetic solid becomes diamagnetic 3 Fe3O4 is example of ferrimagnetic solid 4 Diamagnetic solids get weakly repelled in magnetic field

Physical Chemistry
Atomic StructureA mass spectrum is the plot of O O O O 1 point relative abundance of ions against percentage of ions percentage intensity of ions against their mass charge ratio relative abundance of ions against their mass charge ratio relative abundance of ions against number of atoms present

Physical Chemistry
GeneralAn ideal gas initially at temperature pressure and volume 27 C 1 00 bar and 10 respectively is heated at constant volume until pressure is 10 0 bar it then undergoes a reversible isothermal expansion until pressure is 1 00 bar what is the total work W during this process AMU ENGG 2018 a 23 02 x 10 J c 14 0 x 10 J b 14 0x 10 J d zero

Physical Chemistry
GeneralWrite the chemical equation of complex compound formation when the following substances are mixed Cu NO3 2 NaSCN in excess Explain and write the chemical reaction equations on what will happen with the obtained complex compound in a solution if the following substances are added a HNO3 solution b K S solution

Physical Chemistry
GeneralChoose the correct statement 1 point out of the following O O Conjugation shifts emission to red and greatly increases quantum yield Halogens lead to sharp decreases in quantum yield Structural rigidity enhances fluorescence All of the above

Physical Chemistry
EnergeticsFor the process H O 1 1 bar 373 K H O g thermodynamic 1 bar 373 K the correct set of parameters is AG 0 AS ve a C AG ve AS 0 b d AG 0 AS ve 2007 3M AG ve AS ve

Physical Chemistry
Equilibriumd P is the ratio of the concentrations of products to the concentration of reactants present in a reaction mixture when chemical equilibrium is reached 1 point Consider the following reaction Choose the expression best describes the equilibrium constant 1 point For the reaction A 2B 2C the following concentrations were obtained for this reaction at equilibrium A 0 200 M B 0 400 M and C 3 50 M Determine the value of K for this reaction at these conditions Disregard units in your answer 1 point A reaction that yields mostly products has a K 1 point Aroaction

Physical Chemistry
GeneralAssertion A During chemical reaction ato ms of one element do not change into those of another element nor disappear from the mix ture Reason R As chemical reaction involves th e breaking and making of bonds between atoms to produce new substance Give the answers and tell if the reason is the

Physical Chemistry
EnergeticsIn a closed insulated container a liquid is stirred with a paddle to increase its temperature In this process which of the following is true CBSE PMT 2002 a AE W Q 0 c AE W 0 Q 0 For th action b AE 0 Q W 0 d AE Q 0 W 0

Physical Chemistry
GeneralC in a sample can be obtained using indirect titration All carbon compounds are converted to CO gas The CO gas is then reacted with excess Ba OH 2 See reaction below CO2 g Ba OH 2 aq BaCO3 s H O The excess Ba OH 2 is reacted with HCl What type of titration is involved in this analysis a acid base b complexometric C precipitation d rodov

Physical Chemistry
EquilibriumCalculate the degree of ionization of Example 7 7 pyridine CHN in its 0 1 M solution K for pyridine is 1 5 x 10 What would be the degree of ionization of pyridine if the solution is also 0 1 M in NaOH

Physical Chemistry
GeneralWhat is the major organic product formed in the following reaction CH3 HC C Na A H C CH3 H3C Br CH3 CH HCCH B H C CH CH3 CH NaBr Tagge Scie Alka

Physical Chemistry
EquilibriumA buffer solution that is 0 440 M in HNO and 0 440 M in NaNO has a pH of 3 35 Addition of which of the following would increase the capacity of the buffer for adde OH Select all that apply NaNO both HNO2 and NaNO simultaneously HNO pure water o of the ohoua

Physical Chemistry
GeneralA thin glass bulb of 100 mL capacity is evacuated and kept in 2 0 L container at 27 C and 800 mm pressure If the bulb implodes isothermally calculate the new pressure in the container in kilopascals kPa

Physical Chemistry
EquilibriumH 0 CH4 CO C H O CO CH4 C H O CO CH4 II K IV K Consider the following reaction Choose the expression best describes the equilibrium constant CO CH4 C H O Describe equilibrium constants CO CH4 C H O A I B II C III D IV

Physical Chemistry
GeneralChoose the correct one out of 1 point the following O O O O Lower the viscosity of solvent lower the quantum yield Lower the viscosity of solvent higher the quantum yield Viscosity has no effect over quantum yield higher the viscosity of solvent higher the quantum yield

Physical Chemistry
Gaseous and liquid statesCalculate the pressure exerted by 1023 gas molecules each mass 10 g in litre The rms velocity is 105 cm sec L A 3 33 x 10 dyne cm C 3 33 x 10 dyne cm What must be the temperature A 3614 8 K B 3 33 x 105 dyne cm D 3 33 x 10 dyne cm B 2214 8 K P What is the total kinetic energy in cal of these particles A 1175 0 Cal B 1195 0 Cal C 1155 0 Cal D 1185 0 Cal 3 PV 3x3 33x107x10 D 2414 8 K 2 P 1 C 1214 8 K I mnc Ixlx 25 1000 T K 6 022 11 8 314x TX107

Physical Chemistry
EquilibriumExample 7 6 Calculate the degree of ionization of 0 02 M acetic acid if its K 1 8 x 10 5 What would be the degree of ionization if the solution also contains 0 01 M sodium acetate CH COO aq H ag 100H a

Physical Chemistry
Solid statePASSAGE Miller introduced a system to designate a plane in a crystal Miller indices group of three numbers that indicates the orientation of a plane or set of parallel planes of atoms in a crystal Read the following passage to answer the give options Marks 1 The miller indices of a plane which intercepts at ab 2 30 OPTIONS 421 361

Physical Chemistry
Equilibrium55 Consider the following reactions C s O g CO g x kJ CO g O g CO g y kJ The heat of formation of CO g is 1 x y kJ mol 2 x y kJ mol 3 y x kJ mol 4 None of these

Physical Chemistry
GeneralMark the correct statement MP PET 1997 a For a chemical reaction to be feasible AG should be zero b Entropy is a measure of order in a system c For a chemical reaction to be feasible AG should be positive d The total energy of an isolated system is constant ustom contains

Physical Chemistry
General1 In group 16 the ve value of AH is the lowest for oxygen eg 2 Chlorine has a higher ve AH than that of F eg 3 He has the highest AH and the highest AH amongst all elemen eg 4 A H of Cs is more ve than that of TI

Physical Chemistry
GeneralWhich of the following thermochemical equations have values of AH rxn that correspond to the enthalpy of formation AH 3H g N g 2NH3 g O2H2 g O2 g H O e N2 g O2 g NO2 g 2NO g O2 g 2NO2 g OH O 202 H 0 0
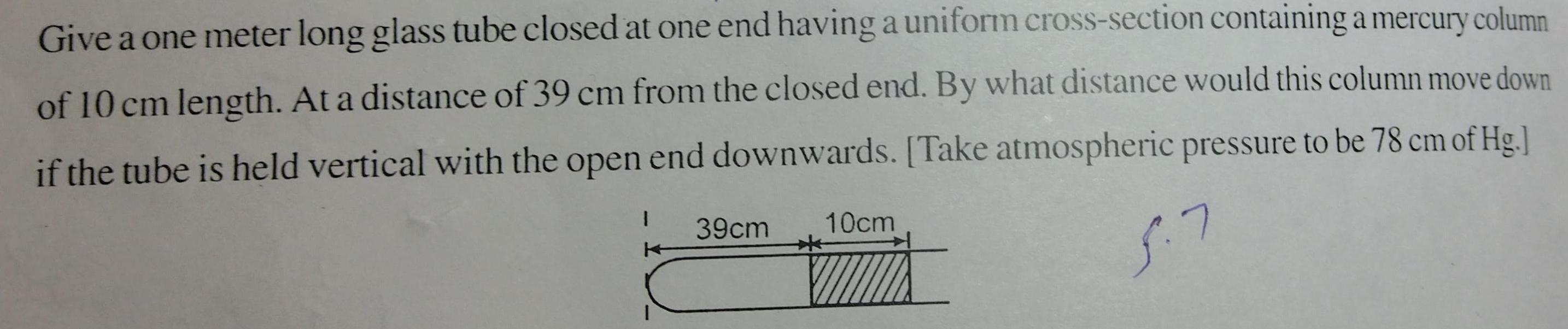
Physical Chemistry
GeneralGive a one meter long glass tube closed at one end having a uniform cross section containing a mercury column of 10 cm length At a distance of 39 cm from the closed end By what distance would this column move down if the tube is held vertical with the open end downwards Take atmospheric pressure to be 78 cm of Hg 5 7 1 39cm 10cm E

Physical Chemistry
GeneralWhich representation best corresponds to an aqueous solution originally containing each of the following Drag the correct picture to match each compound Answers may be used more than once a b C d 1 M CH3CO H 1 M NaOH 1 M NH3 1 M HCI 1 M Na CO3 1 M H SO4 1 M CaCl 1 M H PO4

Physical Chemistry
GeneralThe PMR spectrum of compound shows following signals 1H NMR 1H singlet 2 3 d 2H triplet 3 6 6 2H sextet 1 55 6 3H triplet 0 9 6 What is the correct structure of the compound O CH3 2CHCH2OH O CH3COOCH2CH2CH3 O CH3COCH2CH2CH3 1 pc

Physical Chemistry
GeneralAt 25 C the density of a propanol water C3H OH in H O solution which is 30 by mass propanol is 1 05 g cm Given that the Partial Volume of water in this solution is 16 8 m mol calculate the Partial Molar Volume of propanol in the solution

Physical Chemistry
Atomic Structure5 i The wavelength of the first line of Lyman series of hydrogen atom is 1215 Calculate the wavelength of the second line of the series and the series limit 6 ii A bike rider approaching a vertical wall observes that the frequency of his bike

Physical Chemistry
SolutionsM 0 If 300 mL of HCI is mixed with 100 mL of 10 M Ca OH 2 then nature of resulting solution 10 and molarity of excess of reactant left is 1 Basic 0 1 M 3 Acidic 0 25 M 2 Acidic 0 025 M 4 Basic 0 01 M

Physical Chemistry
Solid stateThe N el temperatures of the ortho ferrite perovskites LnFeO3 are listed below Hint These are all orthorhombic perovskites SYC Lecture 4 p25 of the pdf how would you expect this distortion to effect the Fe 0 Fe angle and how will this be effected by changing the tolerance factor Compound N el Temperature K LaFeO3 743 PrFeO3 707 NdFeO3 SmFeO3 EuFeO3 GdFeO3 689 673 662 658 n terms of superexchange explain why FeF3 has a significantly lower N el temperature 200 K than LaFeO3

Physical Chemistry
EquilibriumAn analytical chemist is titrating 181 0 mL of a 0 7700M solution of propionic acid HC H CO with a 0 3100M solution of NaOH The pk of propionic acid is 4 89 Calculate the pH of the acid solution after the chemist has added 335 4 mL of the NaOH solution to it Note for advanced students you may assume the final volume equals the initial volume of the solution plus the volume of NaOH solution added Round your answer to 2 decimal places

Physical Chemistry
Chemical Bondingnich of the following combinations would form a solution 1 Water and ethanol II Sand and table salt III Oxygen and nitrogen IV Oil and vinegar A I B II C III D II and IV E I and III

Physical Chemistry
EnergeticsCalculate A H for the reaction BonREDMI NOTE 14 enthalpAQUAD CAMERA 1 11 H H L C C 30 0 The average bond enthalpies of various bonds are Bond C H 0 0 C O 499 HH 724 20 C 0 2H 0 H O H 460 C C 619

Physical Chemistry
Chemical kineticsTime and concentration data were collected for the reaction A products 0 20 40 60 80 100 A M 0 52 0 43 0 35 0 29 0 23 0 19 The blue curve is the plot of the data The straight orange line is tangent to the blue curve at t 40 s Approximate the instantaneous rate of this reaction at time t 40 s instantaneous rate M s 0 52 0 48 0 44 0 40 0 36 0 32 0 28 0 24 0 20 0 16 0 10 20 30 40 50 60 70 80 90 t s

Physical Chemistry
GeneralA water source has a flow of 10 MGD alkalinity of 275 mg L in CaCO3 and a pH of 8 85 What will the pH be after addition of 150 mg L NaOH after it has been given adequate time to reach equilibrium with the atmosphere ie open system

Physical Chemistry
GeneralWhich of the following is not needed to use uv vis absorption spectrometry as a quantitative technique a The sample has to be in a solution form b The sample does not contain other molecules that absorb in wavelength used for analysis C The compound that will be monitored obey s Beer Lambert s law d The compound that will be monitored has only one Lambda max wavelength with maximum absorption

Physical Chemistry
EnergeticsMass and energy are conserved is demonstrated by MH CET 2 a First law of thermodynamics b Law of conservation of energy c Law of conservation of mass d Modified form of Ist law of thermodynamics

Physical Chemistry
ElectrochemistryWhich is a redox reaction 2Cul 2Cul 1 NaCl AgNO3 AgCl NaNO3 NH4Cl NaOH NH3 NaCl H O Cr SO4 2 6KOH2Cr OH 3K SO

Physical Chemistry
GeneralA sample of 3 674 g of sulfur dioxide is dissolved in enough water to make a total solution volume of 250 mL What is the molarity of this solution Your units are understood to be M Please only answer numerically

Physical Chemistry
Chemical kineticsIn cv y 0 03212 slope 1st order time min 0 03212 x 10 85 units Kun I in cv in cv y 0 0 2348 slope 234 8 2nd order time min X 4 397x10 units

Physical Chemistry
Equilibrium5 2 points Consider a saturated solution of calcium oxalate CaC 04 CaC O4 s Ca aq C 042 aq CO C 02 H O HC O4 aq OH aq HC 04 H O H C O4 aq OH aq K p 1 3 x 108 Kb 1 8 x 10 10 Kb 1 8 x 10 13 If oxalic acid H C O4 is added to the saturated solution of CaC204 would each of the followin increase decrease or remain the same a pH b solubility of CaC 04 c K of C 04 d Ca in solution

Physical Chemistry
Chemical kineticsa For the following consecutive reaction if k k2 prove that the rate of the reaction depends on the step 2 assume the reactions follow first order kinetics k k step 1 step 2 b What is temperature coefficient for a reaction X Y Z

Physical Chemistry
Chemical kineticsQuestion 4 of 36 Time and concentration data were collected for the reaction A products t s 0 20 40 60 80 100 A M 0 52 0 43 0 35 0 29 0 23 0 19 The blue curve is the plot of the data The straight orange line is tangent to the blue curve at t 40 s Approximate the instantaneous rate of this reaction at time t 40 s instantaneous rate 0 00875 M S 0 52 0 48 0 44 0 40 0 36 0 32 0 28 0 24 0 20 0 16 0 10 20 30 40 50 60 70 t s At

Physical Chemistry
GeneralThe rate constant for this first order reaction is 0 930 s at 400 C A products How long in seconds would it take for the concentration of A to decrease from 0 610 M to 0 340 M t