Energetics Questions and Answers

Physical Chemistry
EnergeticsQ17 Identify which of the following statement is True O A In the case of an ideal gas isothermal irreversible work of expansion is greater than that of reversible work of expansion O B All the natural processes are irreversible O c Density is an extensive property O D If in a process H H final initial the process must be exothermic

Physical Chemistry
EnergeticsWhich of the following statement is 1 point TRUE O O O Particle following F D static are distinguishable Particle following F D static are indistinguishable Particle following B E static are distinguishable Particle following M B static are indistinguishable

Physical Chemistry
EnergeticsC H OH g CH CHO g H g AH 60 kJ If the molar ratio of C H g to CH CHO g is x y in the above process and 52 kJ is absorbed when 1 mol of C H5OH dissociate according to above reaction Then 1 0 25 3 4 X C H g H O g AH 50 kJ y is 2 2 4 8

Physical Chemistry
EnergeticsA reaction is non spontaneous when placed in ice water but spontaneous when placed in a hot bath What can you deduce regarding the signs for AH and AS for this reaction A Both are positive B AH is positive and AS is negative C AH is negative and AS is positive D Both are negative

Physical Chemistry
EnergeticsIf air contains 20 by volume O and 80 by volume N the ratio of mole fractions of N to O dissolved in water at 25 C from air is K 0 3 25x10 and K N 6 5x 10 atm Answer 00 01 02 03 04 05 06 07 08 09

Physical Chemistry
Energetics1 lodine molecule dissociates into atoms after absorbing light of 4500 If one quantum of radiation is absorbed by each molecule the kinetic energy per iodine atom is given by x 10 J in scientific notation What is the value of x ly Use h 6 6 x 10 34 Js c 3 x 108 ms and N 6 x 1023 bond energy of 1 240 kJ mol

Physical Chemistry
EnergeticsWhen hydrochloric acid is added to cobalt nitrate solution at room temperature the following reaction takes place and the reaction mixture becomes blue On cooling the mixture becomes pink Co H O aq 4C1 aq Pink CoC141 aq blue 6H O l On the basis of this information mark the correct answer a AH is positive for the reaction b AH is negative for the reaction c AH 0 for the reaction d The sign of AH cannot be predicted on the basis of A

Physical Chemistry
Energetics2 moles of ideal gas is expanded reversibly from 4 atm to 3 atm at constant temperature of 300 K Calculate the work done approx 1 273 cal 3 402 cal log4 0 6 log3 0 48 2 332 cal 4 315 cal

Physical Chemistry
Energetics3 A mixture of two miscible volatile ideal liquids P and Q obeying Raoult s law is kept in a vessel molar ratio of P and Q in the mixture is m At a suitable temperature T the vapour above the liquid is condensed in another vessel The liquid obtained on con densation is allowed to evaporate and establish equilibrium with its vapour The vapour is then condensed in another vessel The process of such evaporation and condensation is repeated for n times If the ratio of the vapour pressure of pure P to that of pure Qis p the molar ratio of P and Q in the condensed liquid obtained after nth cycle for finite n 1 is A p m C pn 2mn 2 B pm D p mn

Physical Chemistry
EnergeticsAn ideal gas undergoes through following cyclic process 1 2 Reversible adiabatic compression from P V T to P V T2 2 3 Reversible isochoric heating from P2V T2 to P3V3T3 3 4 Reversible adiabatic expansion from P3V3T3 to P4V4T4 4 1 Reversible isochoric cooling from P4V4T4 to P V T Efficiency of the cycle is Question Type Multiple Correct Type 1 2 3 1 3 T 17 12 2 T 1 T4 T T3 T2 1 r 1 1

Physical Chemistry
EnergeticsThe solubility of BaSO4 in water is 2 42 10 3 gL 1 at 298 K The value of its solubility product Ksp will be Given molar mass of BaSO4 233 g mol 1 NEET 2018 A B C D 1 08 10 10 mol L 2 1 08 10 12 mol L 2 1 08 10 8 mol L 2 1 08 x 10 14 mol L 2

Physical Chemistry
EnergeticsAlgen 57 32 841 016 NH Ha S OH 14 143 04 1 1 0 286 2 SH OH AH 46 228 88 KJ 46 K 436 kJ 3H NH s will Jord 16th 1 142 X 112 Kind s avg Gond enthalpy if 352 45 X 3 436 3m taxi 450

Physical Chemistry
EnergeticsThe standard enthalpy of formation of H O g at 300 K is 57 8 kcal mol Calculate AH at 400 K Given Cp H O g 7 2 5 x 10 T Cp Hg g 7 2 x 104T Cp O g 6 4x103T Cp are measure in units of cal K mol 1 58 08 kcal 2 50 6 kcal 2 333 3 kcal 4 7131 2 cal

Physical Chemistry
EnergeticsConsider the following reaction 2Fe2O3 s 3C s 4Fe s 3CO g AH of Fe O3 CO2 are 820 kJ mol and 390 kJ mol respectively The reaction is Endothermic Exothermic Spontaneous at high temperature Spontaneous at low temperature

Physical Chemistry
EnergeticsHow much heat is required to raise the temperature of 1 mole of oxygen gas from 27 C to 127 C at 1 atm pressure Given Cp 6 3 25 x 10 3 T 1 x 10 6 T2 cal K 1 mol 1 0 7 kcal 3 2 8 kcal 2 1 4 kcal 4 1 kcal

Physical Chemistry
EnergeticsWhat will be the HTML code for the following output in a web page Diwali symbolises the victory of good over evil

Physical Chemistry
EnergeticsEnter your answer in the provided box At a local convenience store you purchase a cup of coffee but at 98 4 C it is too hot to drink You a 32 7 g of ice that is 2 2 C to the 248 mL of coffee What is the final temperature of the coffee Assu the heat capacity and density of the coffee are the same as water and the coffee cup is well insulated Tec

Physical Chemistry
EnergeticsFor which of the following process entropy of system containing ideal gas increases Single stage adiabatic compression Reversible adiabatic expansion Irreversible adiabatic expansion

Physical Chemistry
Energetics1 4 Vapor pressure of liquid water at 298 K is 3 16 kPa The standard molar Gibbs energy difference between water vapor and liquid water at 298 K AGm 9 AGm 1 in kJ mol is ignore the effect of pressure on liquid A 0 0 B 8 56 C 8 56 D Information is not enough for calculation

Physical Chemistry
EnergeticsTwo moles of an ideal solution is prepared by mixing pure liquids A and B at 27 C The mole percent of A in the vapour above the solution is 60 Vapour pressure of pure liquids A and B are 120 mm of Hg and 80 mm of Hg The free energy change of mixing AmiG of the solution in calories is Take R 2 cal K mol log 2 0 3 log3 0 48 log5 0 7 In10 2 3 AH 0 AS nR xilnxi A 1 1 2 x 2 n n ln 2 2 2 2 AG TAS x2 log 3x2 3x2x2 log

Physical Chemistry
EnergeticsEXAMPLE 42 The rate of reaction doubles when its temperature changes from 300 K to 310 K Activation energy of such a reaction will be R 8 314 J K mol and log 2 0 301 a 48 6 K J mol b 58 5 kJ mol c 60 5 kJ mol d 53 6 kJ mol I I T mains 2013

Physical Chemistry
EnergeticsGiven n 1 n 1 2rn where nth n 1 and n 1 th shell respectively Calculate the value of n The energy of separation of an electron is 30 6 eV moving in an orbit of Li 2 Find number of waves made by the electron in one complete revolution in the orbit Calculate the number of waves made by a Bohr electron in one complete revolution orbit of H atom if ratio of de Broglie wavelength associated with electron moving in n and 2nd orbit is 1 5 A certain dye absorbs lights of 2 400 nm and then fluorescence light of wavelength 5 Assuming that under given condition 40 of the absorbed energy is re emit fluorescence calculate the ratio of quanta absorbed to number of quanta emitted ou A photon of energy 4 5 eV strikes on a metal surface of work function 3 0 eV If uncerta

Physical Chemistry
EnergeticsThe temperature of equal masses of three different liquids A B and C is 12 18 and 28 C respectively When A and B are mixed the temperature is 16 C When B and C are mixed it is 23 C Selected correct statements

Physical Chemistry
Energetics47 Scientist of UK have invented the cars working on hydrogen fuel cells instead of petrol engines Here hydrogen is used as a sources of electrical energy i e a reaction of hydrogen an oxygen is used to generate electrical energy It has many advantages over the conventional fossil fuels and electric power generation i Give two advantages of hydrogen over fossil fuels ii What is the efficiency of fuel cell as comparison to other conventional fuels iii What are the values possessed by scientists of UK

Physical Chemistry
EnergeticsC s O g CO g 94 2 Kcal H g O g CH g 20 g The heat of formation 1 45 9 2 47 8 3 209 72 H O 1 68 3 Kcal CO g 2H O 21 08 of methane in Kcal will be

Physical Chemistry
EnergeticsConsider the following acid catalysed reaction A H B Rate R K H At pH 3 time to reduce the concentration of A from 5 M to 3 5 M is 60 minutes Which of the following statement s is are true 1 The value of K is 25 min The value of K is 25 M min The value of K is 50 M min Half life of the reaction is 100 min

Physical Chemistry
EnergeticsOne mole of an ideal monoatomic gas is taken from state A to state B through the process 1 T It is found that its temperature increases by 115 2 K in this process Now it is taken P 3 2 from state B to C through a process for which internal energy is related to volume as U The volume at B is 100 m and at C it is 1600 m then the total work done by the gas is ax 10 2 Then the value of a is R 25 3 J mol K

Physical Chemistry
EnergeticsA piece of lead of mass 1 kg at 50 C is placed in a copper container of mass 50 g containing 200 g of liquid X at 20 C If the final temperature of the system is 30 C predicting the specific heat capacity of the liquid X Given that the specific heat for copper and lead are 390 J kg C and 130 J kg C 1 respectively

Physical Chemistry
Energeticsgm H O A is mixed with 70 gm of H O B in a rigid adiabatic vessel The initial temperature of A B was 46 70 C Fir ange in enthalpy of water H O A in calories specific heat of water 1 cal gm c

Physical Chemistry
EnergeticsN 46 48 49 50 The Enthalpy of formation of H SO4 at 298K will be Given S 02 SO SO2 1 2 0 SO3 SO3 H O H SO4 H 1 2 02 H O Question Type Single Correct Type 1 810 KJ 3 1 2 710 KJ 4 51 52 53 54 55 56 680 KJ English Review Q 510 KJ AH 300 KJ AH 100 KJ AH 10 KJ AH 280 KJ

Physical Chemistry
EnergeticsNitrogen and Carbon dioxide gases are heated from 800K to 1300K at a constant pressure of 1 bar For heating of the two gases which process will be more spontaneous Note Comment using proper calculation only Answer without calculation will not be justified You may use required parameters constant for solving from any external source Please mention the data equation used for your calculation

Physical Chemistry
EnergeticsThe preparation of SO3 g by reaction SO g O g SO3 g is an exothermic reaction If the preparation follows the following temperature pressur relationship for its yield then for temperatures T T2 and T3 The correct option is PEA 40 20 10 Pressure atm O T3 T T1 O T1 T T3 O T T T3 O Nothing could be predicted about temperature through given information Marks 4 1

Physical Chemistry
Energeticsc A piston expands against latm of pressure fro absorbed Consider a mixture of air and gasoline vapour in a cylinder with a piston The original volume is 40 cm If combustion of this mixture releases 950J of energy to what volume will the gas in L expands against a constant pressure of 5 bar if all the energy of combustion is converted into work to push back piston CIL bes a volume of 876 L at 0 08 C and 1 atm pressure At constant balloon to expand to a volume

Physical Chemistry
EnergeticsAn electron jumps to higher excited state of a orbital which is non directional and have 4 radial node then shell with which electron belong Magnetic momentum of an ion M3 is 35 BM Then number of electron in d orbital of M element will be A sample contain only hydrogen atoms and in all atom electron are present at 4th excited state How many minimum number of atoms required if total seven lines are observed in emmission spectrum

Physical Chemistry
EnergeticsThe Enthalpy of formation of H SO4 at 298K will be Given S O SO SO2 1 2 O2 SO3 SO3 H O H SO4 H 1 2 O2 H O O 810 KJ O 710 KJ O 680 KJ O 510 KJ AH 300 KJ AH 100 KJ AH 130 KJ AH 280 KJ

Physical Chemistry
Energetics4 Enthalpy 13 10 mol of ideal gas expanded reversibly isothermally from 1 L to 10 L at 100 K The work done during the process is NCERT Pg 166 1 1 91 kJ 3 19 1 kJ 14 If ArH of A B B C and A D are x y and 7 kJ mol 1 respectively then of AMERA 2 0 95 kJ 4 38 2 kJ L 2 2 CO 6 IM 4

Physical Chemistry
Energetics1 mole ideal gas undergoes change in state from 2 atm 5 litre 300K to Pf Vf Tf P If w 6 93 atm litre Q If w 5 atm litre 1 2 R If w 30 atm litre 3 Process is irrreversible isothermal at Pext 1 atm Process is isobaric at 2 atm pressure with V 20 litr Process is reversible isothermal with 1 atm

Physical Chemistry
Energetics60 1 mole of an ideal monoatomic gas initially at 1 atm and 300 K experiences a process by which pressure is doubled The nature of the process is unspecified but AU 900 cal The final volume will be 1 Given R 0 08 atm lit mol K 2 Cal K mol J

Physical Chemistry
EnergeticsQ 57 3 5 g of a fuel with molecular weight 28 was burnt in a calorimeter and raised the temperature of 1 g water from 25 C to 67 3 C If all the heat generated was used in heating water the heat of combustion of fuel is k cal
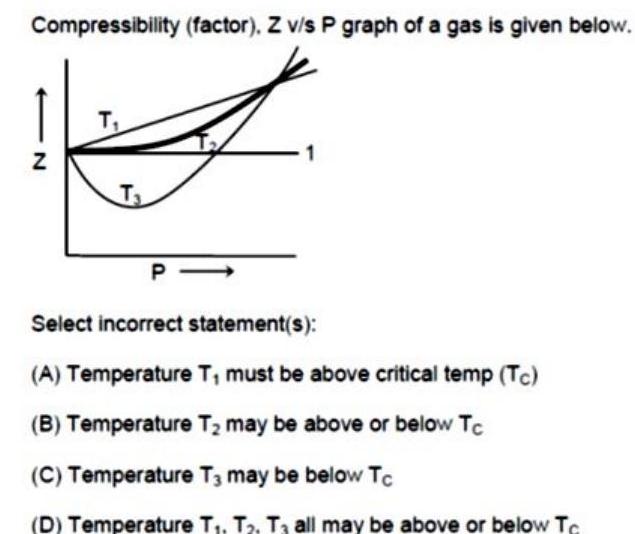
Physical Chemistry
EnergeticsCompressibility factor Z v s P graph of a gas is given below Z T T P Select incorrect statement s A Temperature T must be above critical temp Tc B Temperature T may be above or below Tc C Temperature T3 may be below Tc D Temperature T T T3 all may be above or below Tc

Physical Chemistry
EnergeticsChemistry Section A B If a gas absorbs 200 J of heat and expands by 500 cm 3 6 fe 2002 x 10 Nm 2 from against a constant pressure 2 x 10 Nm2 then change in f 500 cm af dt make a Internal energy is 1 300 J 2 100 3 100 J 4 300 J The rate law for reaction A B C Dis rate K A11 2 B 2 If the reaction aftada t 1 300 J 2 100 J 3 100 J 4 300 J 7 ff A B C D fant

Physical Chemistry
EnergeticsE X g E2 Xig X g Column l Steps P E Q E R E3 E3 X g Column II Energy involve in given step 1 IEA of X g l 2 IEA of X g l 3 IIE of X g l E4 PQRS 2314 S E4 4 IEA of X g l Select correct code for your answer 1 2 32 1 4 3 23 4 1 4 2 1 3 4 Xig

Physical Chemistry
EnergeticsDecomposition of H O is shown below 2 H O 1 2 H O l O g At the end of the reaction 0 75 L of gas was collected over water at 25 C and the total pressure measured was 766 mm Hg How many moles of H2O2 were consumed by the reaction Given vapor pressure of water at 25 C 24 mm Hg 1 atm 760 mm Hg OA OB OC OD R 0 0821 atm L mole K 766 760 0 75 0 0821 2 25 273 15 766 24 760 0 75 2 0 0821 25 273 15 766 24 760 0 75 2 34 0158 0 0821 25 273 15 34 0158 0 75 25 273 15 H O 34 01 58 g mole

Physical Chemistry
Energeticsat equivalent point is pK of A 8 log2 0 3 At 298 K bond dissociation enthalpies of C C and C C are 330 and 540 KJ mol respectively If th enthalpy of polymerisation per mole of polyethene C H g from ethene gas at 298 K is 6MJ th value of n is

Physical Chemistry
EnergeticsI have learnt that internal energy U is a state function and it only depends on temperature So if Delta T 0 then Delta U 0 However when I was studying exothermic and endothermic reactions it was written in my textbook that at a constant temperature and pressure Delta U is negative for exothermic reactions How is that possible Shouldn t Delta U be zero since the temperature is constant

Physical Chemistry
EnergeticsConsider the following setup of two bulbs with adiabatic walls separated by an adiabatic valve The left side flask is of volume 1L and contains 0 2 moles of N O4 and 0 1 moles of NO at equilibrium at 25 C N O g 2NO g The larger flask on the right side is of 3L volume and is empty at 25 C The connecting valve is suddenly opened The correct statement s regarding this system is re A Total number of moles after opening the valve is 0 34 B Entropy of the system keeps on increasing till the system regains the equilibrium C The total number of moles remain the same before and after opening of the valve D Ke value for the equilibrium is 20

Physical Chemistry
EnergeticsO The composition of Liquefied Petroleum Gas LPG is 0 5 ethane 0 1 acetylene 16 4 propane 2 1 ethylene 74 butane and 6 9 butene If the room temperature is at 30 C what is the pressure inside the tank and the composition of the gas that first issues from this mixture

Physical Chemistry
EnergeticsHeat transfer is very important for life s progress as everything needs to gain or lose heat even human beings In the process industry the temperature of input and output streams of any equipment should be adjusted by exchanging the heat between fluids Different types of Heat exchangers are used in process industries for this purpose such as shell and tube heat exchangers Analyze the diagram of the shell and tube heat exchanger shown below to answer the associated questions h PRO b i Heat is transferred by three different methods use your knowledge by explaining the methods of transferring heat and show how it 2 Marks is applying in a heat exchanger

Physical Chemistry
Energeticsbenzer i mole HEMISTRY The The combustion of takes place at 21Sk flatm After combustion CO g and H O 1 are produced and 3267 0 kJ of heat is liberated Calculate the standard enthalpy of formation A H of benzene Standard enthalpies of formation of CO g and H O 1 are 393 5 kJ mol and 285 83 kJ mol respectively

Physical Chemistry
Energetics5 In comparison to a 0 01 M solution of glucose the depression in freezing 1 poin point of a 0 01 M MgCl2 solution is O The same O About twice O About three times O About six times 4