General Questions and Answers

Physical Chemistry
GeneralPak 77 CAREER INSTITUTE MOTA HAIMITRIN An ideal gas undergoes through following cyclic process 1 2 Reversible adiabatic compression from P V T to P V T 2 3 Reversible isochoric heating from P V T to P V T 3 4 Reversible adiabatic expansion from P V Tato PVT 4 1 Reversible isochoric cooling from PVT to P V T Efficiency of the cycles is A 1 B 1 D 1 Q4 11 Q C 1 78 An ideal gas initially at PV T is expanded isothermally to twice it s volume then compressed at constant pressure to have the original volume V Finally the gas is heated at constant volume to get original temperature what is correct A P V diagram for the process is B Total heat supplied is 0 193 PV C Net work is done by the gas D Interal energy remain same during the who 79 The spontaneity means having the potential to proceed without the assistance of external a hec

Physical Chemistry
GeneralThe molar conductance of a strong electrolyte at infinite dilution A tends to a finite value which is above that a higher concentration B tends to a finite value which is below that at higher concentration C tends to zero D tends to a finite value which is equal to that at high concentration

Physical Chemistry
General13 2 Moles of potassium reacts with cold water to give x Moles of hydrogen and 3 moles of iron reacts with steam to give y moles of hydrogen The value of y 2 x will be Only One Correct Answer Review A 8 O

Physical Chemistry
GeneralIn a particular system the half life of Lindane undergoing aerobic decomposition is 210 days A contaminated groundwater has a Lindane concentration of 30 ppb The maximum contamination level MCL for Lindane is 0 1 ppb How long does it take for the concentration of Lindane to be below MCL Do you expect that Lindane will have strong adsorption on solid Calculate its BCF knowing that log Kow is 3 38

Physical Chemistry
GeneralConsider the reaction between Fe and NCS ions The equilibrium constant for this reaction is 620 4 Calculate the equilibrium concentration of NCS ion in a solution that contains 10 0mL of 0 05 M ferric nitrate in 1M HNO3 2 0mL of 5 0 10 M NaNCS and 8 0mL of distilled water Assume that all the NCS is converted to FeNCS 4 A 6 07x10 8 M B 3 04x10 6 M C 0 M D 1 00 x 10 6

Physical Chemistry
GeneralIn the plasma membrane Glycolipids are usually situated in O O cannot be predicted it varies according to the cell types O O 1 point O inner leaflet of plasma membrane O outer leaflet of plasma membrane evenly distributed in both outer and inner leaves of plasma membrane

Physical Chemistry
General1 Common salt obtained from sea water contains 95 NaCl by mass The approximate number of molecules present in 10 0 g of the salt is a 10 1 b 1022 c 1023 d 1024

Physical Chemistry
GeneralA water sample contains 10 6 M copper It has a pH of 7 5 and a CT CO3 of 7 72 x 10 M Assume that CuCO30 aq is the only important Cu complex Cu CO3 CO3 CUCO3 aq log K 6 77 For the carbonate system at pH 7 5 do 0 06630 a 0 9372 and a2 0 001382 a1 The concentration of Cu2 M in solution is

Physical Chemistry
GeneralWhich one of the following statements is not true regarding lactose a Lactose C12H22O11 contains 8 OH groups b On hydrolysis lactose gives equal amount of D glucose and D galactose c Lactose is a B glycoside formed by the union of a molecule of D glucose and a molecule of D galactose d Lactose is a reducing sugar and does not exhibit mutarotation

Physical Chemistry
GeneralPhotoelectric emission is observed from a surface for frequencies v and v of the incident radiation v v If the maximum kinetic energies of the photoelectrons in the two cases are in the ratio 1 k then the threshold frequency vo is given by b a c V2 V1 k 1 kv V1 k 1 d kv V2 k 1 V2 V1 k

Physical Chemistry
GeneralCalculate the equilibrium constant for the reaction 3Mg 2Al 3Mg 2Al For Mg 2e Mg k E 2 363 For Al 3e Al k E 1 662 Cr solution is obtained by electrolysis of Cr solution In 500 mL of 0 15 M Cr solution how long would it take to reduce Cr to Cr using a current of 0 158 A The potential of the DKE MgA2 Mg2 9 62 x 10 M Mg ISE ion selective electrode Cell is 0 367V When the Mg solution in the cell is replaced with an unknown Mg solution the cell potential becomes 0 244 V What is the concentration of this Mg solution

Physical Chemistry
GeneralIn the given process which oxidation state is more stable MIE 7 9 eV M M 42 M2 IE 15 5 eV 1 M The electronic configuration of some neutral atoms are given below 1961 2 M 2 3 Both

Physical Chemistry
GeneralWhat is the product of 1 and 4th reactions Which reaction does not produce ammonia AH B TH 3I H DUI 3THI P Pfa T a NH4 I KOH b N 3H A Fe Mo c NH4 Cr O d Mr N HCL og

Physical Chemistry
GeneralThe degree of inhibition a by a 1 point inhibitor is competitive obtained from O O O O O measurement of Vmax measurement of the y intercept on a Limeweaver Burke Plot measurement of KM is unrelated to the binding affinity of the inhibitor to the enzyme

Physical Chemistry
GeneralPEI can be degraded by a microbe called Id eonella Sakaiensis 1 What is the relation between Heat co nditions Temperature of the environment a nd the time of degrdation 2 What are the by products of this degr adation process Are they beneficial for th e environment

Physical Chemistry
Generalg 16 Enthalpy change for the reaction 4 Hg 2H is 869 6 kJ The dissociation energy of H H AIPMT Prelims 2011 bond is 1 217 4 kJ 2 434 8 kJ 3 869 6 kJ 134 8 k

Physical Chemistry
GeneralWho among the following scientists is not related to production and detection of electromagnetic waves G Marconi H Hertz min sec J C Bose

Physical Chemistry
GeneralColumn l contains pair of cations and column II contains the basis on which the cations can be distinguished Column l Q1 Pb2 and Bi Q2 Zn2 and Fe Q3 Cu and Ni Q4 Cu2 and A1 Column II A1 Excess NH4OH A2 Excess NaOH A3 dmg A4 NH4OH H S A5 H S HCI

Physical Chemistry
GeneralWhen the following skeletal equation is balanced under acidic conditions what are the coefficients of the species shown 12 Fe 103 Fe Water appears in the balanced equation as a reactant product neither with a coefficient of Enter O for neither Which species is the reducing agent

Physical Chemistry
GeneralA 1 00 L sample of a mixture of methane gas and oxygen measured at 25 C and 740 torr pressure was allowed to react at constant pressure in a calorimeter which together with its contents had a heat capacity of 1260 cal K The complete combustion of the methane to carbon dioxide and liquid water caused a temperature rise in the calorimeter of 0 667 K What was the mol percent of CH4 in the original mixture AH comb for CH g 210 8 kcal mol

Physical Chemistry
GeneralSelect the correct graph representing the following equilibrium if initially equal moles of NH3 and O2 are taken in reaction vessel 4NH3 g 50 g 4NO g 6H O g Conc Conc Conc Conc NH H O Time 0 H O Time NO H O Time 0 0 NO Time NH NO 0 NH H O NH NO

Physical Chemistry
GeneralA new assay method is being evaluated in comparison to an existing gold standard method Repeated runs of the new method show assay points that are closely clustered together However the mean measurement from the new assay is significantly different from the mean measurement of the same samples with the gold standard method Which of the following statements could account for this observation There is likely to be an interfering chemical in the new assay method that is affecting the accuracy of the method There are random errors during performance of the new assay method due to operator error The new assay has very little systematic error None of the above
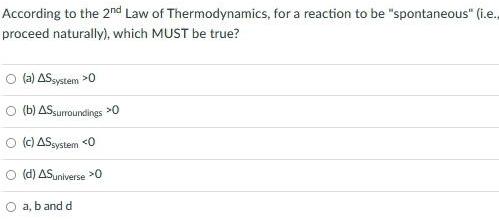
Physical Chemistry
GeneralAccording to the 2nd Law of Thermodynamics for a reaction to be spontaneous i e proceed naturally which MUST be true a AS system 0 b AS surroundings 0 O c AS system 0 d AS universe 0 O a b and d

Physical Chemistry
GeneralQ13 In a regular binary A B solid solution the molar fraction of component B is 0 4 and its activity is 0 5 at temperature of 700 K What is the lowest temperature that can prevent the phase separation in the regular solid solution

Physical Chemistry
GeneralLoamy soil is a mixture of clay silt and san d It is the best top soil for growing plants Which of the following statements most accurately e xplains the property of loamy soil A Holds the maximum amount of water th at enters it B Permits only a certain amoun t of water to enter it C It contains very little amount of humus D Allows water to pass through it rapidly

Physical Chemistry
GeneralA n type semiconductor is obtained if we dope 1 poi O Silicon with a trivalent element O Silicon with a pentavalent element OA pentavalent element with silicon O A trivalent element with silicon

Physical Chemistry
GeneralExample Exercise 17 2 Calculating Oxidation Numbers for Sulfur Calculate the oxidation number for sulfur in each of the following ions a sulfide ion S b c d sulfite ion SO sulfate ion SO thiosulfate ion S 0

Physical Chemistry
GeneralIn the electrolysis of aq NaCl 4 moles of H ape evolved along with 0 5 moles of O Find the mass of Cl liberated CaMAIN BOARD In the electrolysis of aq NaCl 4 moles of H are evolved along with 0 5 moles of O Find the mass of Cl liberated Car MAIN BOARD lo siv of this gu on

Physical Chemistry
GeneralQ8 A solution of 16 hydrochloric acid is to be diluted by adding a 1 hydrochloric acid solution to it The resulting mixture is to be more than 4 but less than 6 hydrochloric acid If we have 1280 L of the 16 solution then how many liters of the 1 solution will have to be added 5

Physical Chemistry
GeneralConsider the following changes 1 kg water AH liquid at 25 C 1 kg water AH liquid at 100 C 1 kg water steam at 100 C Given Specific heat of water 1 cal g C Latent heat of vaporisation of water 10 kcal mo AH is a 75 kcal b 550 kcal c 750 kcal d 55 kcal

Physical Chemistry
General1 The atomic masses of He and Ne are 4 and 20 amu respectively The value of the de Broglie wavelength of He gas at 73 C is M times that of the de Broglie wavelength of Ne gas at 727 C M is a 2 c 4 b 3 d 5

Physical Chemistry
General0 In which of the following reactions H O is not an oxidising agent 2 1 4H O PbS4H O PbSO4 4 Mn 2OH 2 2 Mn H O 3 2FeSO4 H SO4 H O Fe SO4 2H O 4 I H O 2KOH2KI 2H O O

Physical Chemistry
Generalsu 6 The total number of tetrahedral and octahedral voids in 0 5 mol of a compound forming hcp structure are a 6 022 x 1023 6 3 01 x 1023 c 9 033 x 1023 d 12 044 x 1023

Physical Chemistry
General2 For the melting of NaCl heat required is 7 26 kcalmol and AS increases by 6 73 cal mol 1 K 1 The melting point of the salt is 2 500 K 4 1 77 C 1 805 75 C 3 1 77 K

Physical Chemistry
General37 The heat of neutralisation of HCI by NaOH is 55 9 kJ mole If the heat of neutralization of HCN by NaOH is 12 1 kJ mole the energy of dissociation of HCN is 1 43 8 kJ 3 68 kJ 2 43 8 kJ 4 68 kJ

Physical Chemistry
GeneralHigh temperature and low pressure favourable condition for the formation of product in the following reaction aA bB cC then which relation is correct 1 AH ve 2 AH ve 3 AH ve 4 AH ve a b c a b c a b c a b c

Physical Chemistry
GeneralMCB MCB 44214 4 I ofte beni yake to co 11000 e ka switchgear Bu 0244 RACE 15 ofte Apffe BF 1 2314 R P2 Caftro afo Fil 1 pl J r FORT 0 P H 12R J 0 1000 V A mechanical circuit breaker miniature citcuit breaker offee og ofta opi 2 NURIA ARRA OG ER OPG SPOG OKEA I OKER FOR A H H Gente 2 Ci 12 x r ARA ORICE ARKA OG PERO 27rl ok Ch 29 ART H 2 rlh Highest safe current IAR IN P25 Tabs fcc 23 fo for IC ICT OG BEG RIGA 12 pl J r 2 rlh 1 2 Jh P 10 5 A 11 faireen STA SAAR 12 4 2 15 3 4 C F FACTO AGRIC REFR 2 5 2 C C K 220 V S FACT 220 V Corte 3 1 x r 233 1 3 af FruG OR THE 1 3 I MIGE

Physical Chemistry
General30 g of KMnO4 sample containing inert impurity is completely reacted with 60 ml of 56 volume of H O in acidic medium then what is the 2 percentage purity of KMnO4 Molecular weight of KMnO4 158 1 35 5 2 36 8 63 2 CA FO

Physical Chemistry
GeneralConsider a sealed can that is filled with 4 4 kg of water at 115 C and P 248 kPa Now a leak develops and the pressure in the can drops to the local atmospheric pressure of 100 kPa Cp 4 23 kJ kg K The vapor leaves the can as soon as it is formed How much vapour will evapourate before the liquid water reaches its boiling temperature at atmospheric pressure NB water s enthalpy of vapourisation is roughly 2257 kJ kg a 4 400 kg b 0 120 kg c 1 093 kg d 0 030 kg e 0 015 kg

Physical Chemistry
GeneralThe chemical formulae of some acids are listed in the first column of the table below and in the second column it says whether each acid is strong or weak Complete the table List the chemical formula of each species present at concentrations greater than about 10 6 mol L when about a tenth of a mole of the acid is dissolved in a liter of water acid HIO H CO HNO HCI strong or weak strong weak weak strong species present at 106 mol L or greater when dissolved in water X 0 0 Please explain answer and list ALL species present I will be sure to leave

Physical Chemistry
GeneralPeripheral chemoreceptors adjust minute volume a rapidly in response to falling pH of cerebrospinal fluid rapidly in response to changes in blood pH c slowly in response to minor changes in blood pO2 d slowly in response to minor changes in blood pCO2

Physical Chemistry
General62 2 0 mole of PC15 were introduced in a vessel of 5 0 L capacity of a particular temperature At equilibrium PC1 was found to be 35 dissociated into PC13 and Cl The value of K for the reaction a 1 89 PC13 g Cl g PC15 g c 1 33 b 0 377 d 13 3

Physical Chemistry
GeneralAn equi concave lens made up of material of refractive index 1 5 has focal length of magnitude 10 cm The radius of curvature of any surface of the lens will be equal to 5 cm 10 cm 20 cm

Physical Chemistry
GeneralA mixture of xenon and hydrogen gas is compressed from a volume of 100 0 L to a volume of 84 0 L while the pressure is held constant at 93 0 atm Caid the work done on the gas mixture Be sure your answer has the correct sign positive or negative and the correct number of significant digits

Physical Chemistry
GeneralWith the standardized solution what is the corrected established relationship a 10mL Na25203 solution 1232 mg L DO b 10ml Na25203 solution 0 224 mg L DO c 10ml Na25203 solution 1 856 mg L DO d 10ml Na25203 solution 0 104 mg L DO

Physical Chemistry
GeneralFor a chemical reaction at 324K the values of AH and AS are 64 5 kJ and 183 5 J K respectively a Find the value of AG at 324K 10 Pts b If enthalpy and entropy don t depend on the temperature at what temperature the AG is equals to 0 10 Pts

Physical Chemistry
General14 Draw the organic product expected from each of the following reactions Be sure to indicate stereochemistry where appropriate and to include stereoisomers if any 8 pts NaOCH H C H H C Br CH CH3 SN2 CH OH

Physical Chemistry
GeneralVapour density of following reaction at equilibrium X g 3 Y g is found to be 10 when 1 mole of X is taken in 1 L flask The degree of dissociation of X is Atomic mass of X is 30 O 0 5 0 25 O 0 75 O 0 1

Physical Chemistry
GeneralFind the n factor of reactant in the following chemical changes i KMnO4 H Mn2 ii KMnO4 iii KMnO4 OH concentrated basic medium v C O2 CO vii Fe O3 FeSO4 Mn6 iv K Cr O H O Mn H Cr vi FeSO4 Fe O3

Physical Chemistry
GeneralA reaction is run in a bomb calorimeter in which 0 100 mole of X4Y10 is combusted according to the reaction X4Y10 l 6 5 O2 g 4 XO2 g 5 Y 0 e The heat capacity of the calorimeter is 4 2 kJ C and the temperature of the 1 1 L of water in the calorimeter increased in temperature from 25 2 C to 28 3 C Calculate AH at SATP for this reaction and report your answer in kJ mol Density of water is 1 g mL