General Questions and Answers

Physical Chemistry
General9 24 Describe how to prepare 250 mL of 1 00 M ammonia buffer pH 9 00 starting with 28 wt NH3 concentrated ammonium hydroxide listed on the page after the periodic table and concentrated HC1 37 2 wt or concentrated NaOH 50 5 wt

Physical Chemistry
GeneralThe order of energy required to 1 point remove electron is as follows in mass spectrometry is o electrons non conjugated O conjugated non bonding or lone pair of electrons non conjugated conjugated n non bonding or lone pair of electrons o electrons conjugated non conjugated non bonding or lone pair of electrons o electrons non conjugated o electrons conjugated non bonding or lone pair of electrons

Physical Chemistry
GeneralAqueo Will react with aqueous magnesium chloride to give solid silver chloride and aqueous magnesium nitrate If 97 3 g of silver nitrate is allowed to react with 52 0 g of magnesium chloride and 72 4 g of the desired silver chloride is produced what is the percent yield of the reaction Your answer is understood to be a percentage please only answer numerically

Physical Chemistry
GeneralChoose the incorrect 1 Above curie temperature ferromagnetic solid becomes paramagnetic 2 On heating ferrimagnetic solid becomes diamagnetic 3 Fe3O4 is example of ferrimagnetic solid 4 Diamagnetic solids get weakly repelled in magnetic field

Physical Chemistry
GeneralAn ideal gas initially at temperature pressure and volume 27 C 1 00 bar and 10 respectively is heated at constant volume until pressure is 10 0 bar it then undergoes a reversible isothermal expansion until pressure is 1 00 bar what is the total work W during this process AMU ENGG 2018 a 23 02 x 10 J c 14 0 x 10 J b 14 0x 10 J d zero

Physical Chemistry
GeneralWrite the chemical equation of complex compound formation when the following substances are mixed Cu NO3 2 NaSCN in excess Explain and write the chemical reaction equations on what will happen with the obtained complex compound in a solution if the following substances are added a HNO3 solution b K S solution

Physical Chemistry
GeneralChoose the correct statement 1 point out of the following O O Conjugation shifts emission to red and greatly increases quantum yield Halogens lead to sharp decreases in quantum yield Structural rigidity enhances fluorescence All of the above

Physical Chemistry
GeneralAssertion A During chemical reaction ato ms of one element do not change into those of another element nor disappear from the mix ture Reason R As chemical reaction involves th e breaking and making of bonds between atoms to produce new substance Give the answers and tell if the reason is the

Physical Chemistry
GeneralC in a sample can be obtained using indirect titration All carbon compounds are converted to CO gas The CO gas is then reacted with excess Ba OH 2 See reaction below CO2 g Ba OH 2 aq BaCO3 s H O The excess Ba OH 2 is reacted with HCl What type of titration is involved in this analysis a acid base b complexometric C precipitation d rodov

Physical Chemistry
GeneralWhat is the major organic product formed in the following reaction CH3 HC C Na A H C CH3 H3C Br CH3 CH HCCH B H C CH CH3 CH NaBr Tagge Scie Alka

Physical Chemistry
GeneralA thin glass bulb of 100 mL capacity is evacuated and kept in 2 0 L container at 27 C and 800 mm pressure If the bulb implodes isothermally calculate the new pressure in the container in kilopascals kPa

Physical Chemistry
GeneralChoose the correct one out of 1 point the following O O O O Lower the viscosity of solvent lower the quantum yield Lower the viscosity of solvent higher the quantum yield Viscosity has no effect over quantum yield higher the viscosity of solvent higher the quantum yield

Physical Chemistry
GeneralMark the correct statement MP PET 1997 a For a chemical reaction to be feasible AG should be zero b Entropy is a measure of order in a system c For a chemical reaction to be feasible AG should be positive d The total energy of an isolated system is constant ustom contains

Physical Chemistry
General1 In group 16 the ve value of AH is the lowest for oxygen eg 2 Chlorine has a higher ve AH than that of F eg 3 He has the highest AH and the highest AH amongst all elemen eg 4 A H of Cs is more ve than that of TI

Physical Chemistry
GeneralWhich of the following thermochemical equations have values of AH rxn that correspond to the enthalpy of formation AH 3H g N g 2NH3 g O2H2 g O2 g H O e N2 g O2 g NO2 g 2NO g O2 g 2NO2 g OH O 202 H 0 0
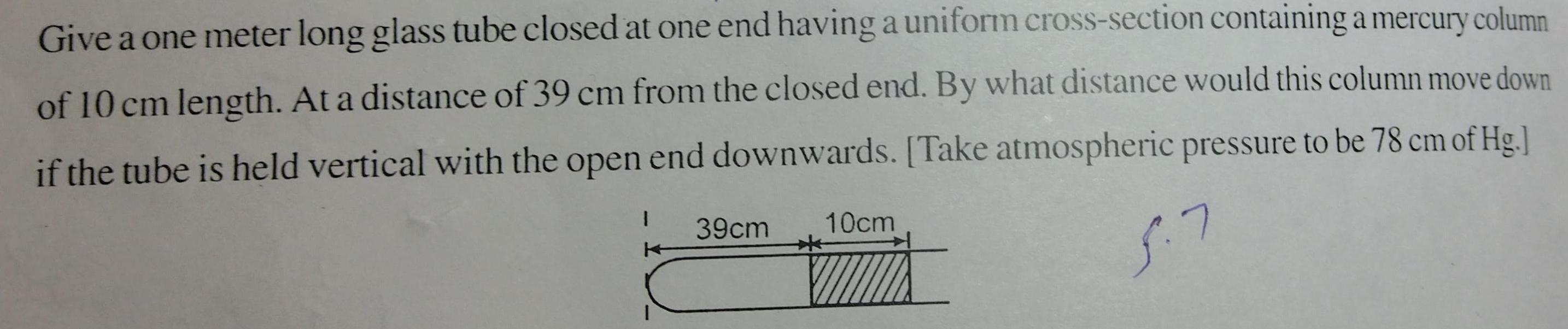
Physical Chemistry
GeneralGive a one meter long glass tube closed at one end having a uniform cross section containing a mercury column of 10 cm length At a distance of 39 cm from the closed end By what distance would this column move down if the tube is held vertical with the open end downwards Take atmospheric pressure to be 78 cm of Hg 5 7 1 39cm 10cm E

Physical Chemistry
GeneralWhich representation best corresponds to an aqueous solution originally containing each of the following Drag the correct picture to match each compound Answers may be used more than once a b C d 1 M CH3CO H 1 M NaOH 1 M NH3 1 M HCI 1 M Na CO3 1 M H SO4 1 M CaCl 1 M H PO4

Physical Chemistry
GeneralThe PMR spectrum of compound shows following signals 1H NMR 1H singlet 2 3 d 2H triplet 3 6 6 2H sextet 1 55 6 3H triplet 0 9 6 What is the correct structure of the compound O CH3 2CHCH2OH O CH3COOCH2CH2CH3 O CH3COCH2CH2CH3 1 pc

Physical Chemistry
GeneralAt 25 C the density of a propanol water C3H OH in H O solution which is 30 by mass propanol is 1 05 g cm Given that the Partial Volume of water in this solution is 16 8 m mol calculate the Partial Molar Volume of propanol in the solution

Physical Chemistry
GeneralA water source has a flow of 10 MGD alkalinity of 275 mg L in CaCO3 and a pH of 8 85 What will the pH be after addition of 150 mg L NaOH after it has been given adequate time to reach equilibrium with the atmosphere ie open system

Physical Chemistry
GeneralWhich of the following is not needed to use uv vis absorption spectrometry as a quantitative technique a The sample has to be in a solution form b The sample does not contain other molecules that absorb in wavelength used for analysis C The compound that will be monitored obey s Beer Lambert s law d The compound that will be monitored has only one Lambda max wavelength with maximum absorption

Physical Chemistry
GeneralA sample of 3 674 g of sulfur dioxide is dissolved in enough water to make a total solution volume of 250 mL What is the molarity of this solution Your units are understood to be M Please only answer numerically

Physical Chemistry
GeneralThe rate constant for this first order reaction is 0 930 s at 400 C A products How long in seconds would it take for the concentration of A to decrease from 0 610 M to 0 340 M t


Physical Chemistry
GeneralUsing either your lecture s textbook or an online resource search solubility curves of ionic compounds what is a possible identity of your unknown compound 378 18 23 16 9 989 Mass of unknown TOTAL MASS H O 9 7 00 mL 7 50 mL 8 00 mL 8 50 mL 9 00 ML 9 SOLUBILITY g solute 100 g H O 0 07 0 075 0 08 0 085 0 09 TEMPERATURE C 67 C 57 C 66 C 64 C 61 C

Physical Chemistry
General78 10cm XPE Figure shows the vertical section of frictionless surface A block of mass 2 kg is released from the position A its KE as it reaches the position Cis A 40 14m Sm B 7m 1 180 J 2 140 J 3 40 J 4 2801 Abody constrained to move in the y direction is subjected 2x10x 114 v 2 X10X7 2 FT 3 7 The pot the x ax V x The total maximur 1 2 2 1 3 2 A body i


Physical Chemistry
General5 68 g of a mixture of CaCO3 and MgCO3 was dissolved in 800 ml of 0 4 M HCI and the solution was diluted to 1 lit 20 ml of this solution was neutralised by 20 ml of 0 1 M Na CO3 The percentage of MgCO3 in the mixture is approx 1 29 6 2 42 910

Physical Chemistry
General31 Ratio of C and C of a gas X is 1 4 The number of atoms of the gas X present in 11 2 litres of it at NTP will be a 6 02 1023 1989 c 3 01 1023 1 c c N O at NTP contains 1 8 a 1022 224 32 6 02 b Pov 22400 1 32 224 c atoms b 1 2 10231 d 2 01 1023 1023 molecules 1023 electrons 1988

Physical Chemistry
General10 The reaction A B follows first order kinetics The time taken for 0 8 mole of A to produce 0 mole of B is 1 hour What is the time taken for conversion of 0 9 mole of A to produce 0 675 m of B a c 0 25 hour 1 hour b 0 5 h d 2 hour

Physical Chemistry
GeneralGive the order of the densities from lowest to highest of the substances shown in the sketch Wood floats on water but sinks in oil oil wood water Suggest a procedure for measuring the density of a liquid Suggest a procedure for measuring the density of a solid that does not have
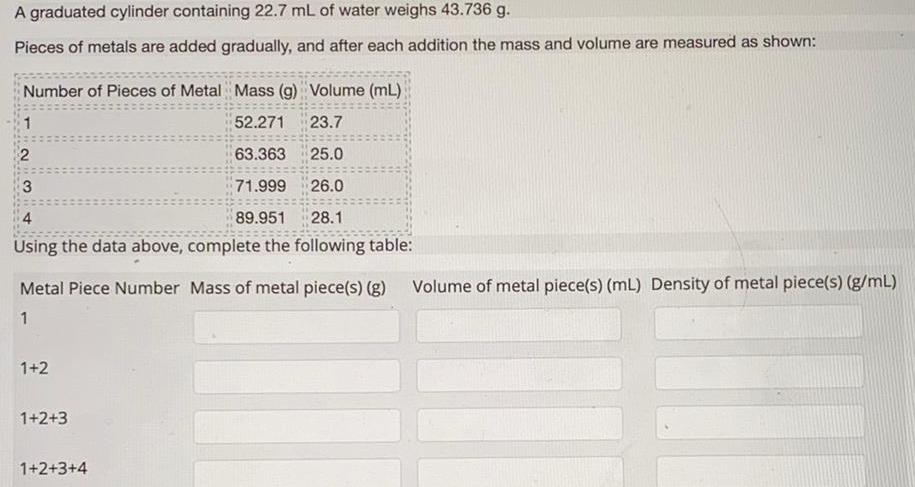
Physical Chemistry
GeneralA graduated cylinder containing 22 7 mL of water weighs 43 736 g Pieces of metals are added gradually and after each addition the mass and volume are measured as shown Number of Pieces of Metal Mass g Volume mL 52 271 23 7 63 363 25 0 3 71 999 26 0 4 89 951 28 1 Using the data above complete the following table 1 2 Metal Piece Number Mass of metal piece s g Volume of metal piece s mL Density of metal piece s g mL 1 1 2 1 2 3 1 2 3 4

Physical Chemistry
General1 0 g of magnesium is burnt with 0 56 g 0 in a closed vessel Which reactant is left in excess and how much 2014 At wt Mg 24 O 16 a Mg 0 16 g c Mg 0 44 g b 0 0 16 g d 0 0 28 g

Physical Chemistry
Generalwith respect to time of flight from the shortest time of night to the longest y m 150 100 75 50 50 60 450 30 for the five path of the figure show 123 fa fa fa v 50 m s 15 100 150 200 Pasino ako T R 250 x m Lampo T sino la sino TAB 1 15 30 45 60 75 2 75 60 45 30 15 3 15 75 30 60 45 4 30 60 15 45 75 A particle is projected from the ground with an initial speed 124 of v at an angle e with horizontal un y m 150 100 75 50 Spartan Batch MEC KTG 2 D Relative Motion fa m after affer 60 459 30 58 m s 15 1 0 0 150 To a sino 1 15 30 45 60 75 2 75 60 45 30 15 3 159 75 30 60 45 4 30 60 15 45 75 200 Tasino Po 250 n sino sk x m Tasino RA 190 30


Physical Chemistry
GeneralFill in the chart about the following particles Charge Location in Atom Protons Neutrons Electrons Job What happens if you change the number of them in atom


Physical Chemistry
General3 1 10 versus time graph on the left The particle s position at t Os is x 10m v m s 10 10 0 5 0 5 x m 54 x m 10 5 4 2 0 2 The diagrams cho Versus time graph goes with the velocity 26 f 494 95 41 When a 2 kg car driven at Gurukripa furug at feafet 0sx 10m 2 5 5 10 10 SI t s t s 10 2 4 10 10 5 0 5 10 x m 10 5 0 5 t s x m 5 10 10 t s t s 1 x m 10 5 0 5 10 x m 10 5 of 5 10 4 2 0 2 v m s 5 5 5 10 t s 10 2 t s 10 4 t s x m 10 5 0 5 10 x m 10 5 0 5 10 5 10 denly put into neutral gear locity decreases in the foll IES VH where t is the time in se instant its speed is 10m 1 1 4 m s 2 1 2 m s 3 1 m s 4 3 4 m s A car is moving unif appears att 0 in fr the driver sees the The following is th stops at a distance t s 1

Physical Chemistry
GeneralScale reading Son who weights w newtons stands on a scale in an elevator that is initially at rest The elevator accelerates up ward to a constant speed and then slows to a stop Which of the following graphs best represents the reading on the scale He 1 Time Scale reading B Scale reading It 3 Scale reading Time Time Time 4 Werft var fore on f for fr Jie cafea shaft fase fieft want man a 2 Scale reading Scale reading Scale reading Scale reading Time Time Time Time and ti frafafena cenfant JE my W a 20 C

Physical Chemistry
GeneralThe rate constant for this first order reaction is 0 0158 s at 300 C A products If the initial mass of A is 18 19 g calculate the mass of A remaining after 1 90 min mass of A


Physical Chemistry
GeneralLeft A long spring is stretched by 2 cm its potential energy is U If the spring is stretched by 10 cm the potential energy stored in it will be 5 2am 4 1 y 10cm sy Figure shows the vertical section of frictionless surface A block of mass 2 kg is m 1 U 25 2 U 5 3 SU 4 25U

Physical Chemistry
GeneralConsider the rate law rate k A Determine the value of x if the rate doubles when A is doubled x Determine the value of x if no change in rate occurs when A is doubled x

Physical Chemistry
GeneralThe half life period of a second order reaction is 30 minutes If the initial concentration is 0 1M the rate constant will be 1 0 666 M x min 2 0 333M x min 3 0 444M x min 4 0 555M x min

Physical Chemistry
General1 The formation of the oxide ion 02 from oxygen atom requires first an exothermic and then an endothermic step as shown below 1 O g e Qg A H 141 kJ mol Piste Oge0 A H 780 kJ mol 8 Thus process of formation of O in gas phase is unfavourable even though O is isoelectronic with neon It is due to the fact that a Orion has comparatively smaller size than oxygen atom b oxygen is more electronegative c addition of electron in oxygen results in larger size of the ion d electron repulsion outweighs the stability gained

Physical Chemistry
GeneralAn assumption for B E T theory 1 point is O All of the mentioned O The adsorption at one site never affects the adsorption at the neighbouring site Physical adsorption of adsorbate is O always occurred resulting in the formation of the multilayers Solid surface possess uniform localised sites

Physical Chemistry
GeneralA metal oxide has the formula Z 03 It can be reduced by hydrogen to give free metal and water 0 1596 g of the metal oxide requires 6 mg of hydrogen for complete reduction The atomic weight of the metal is 1989 a 27 9 c 79 8 b 159 6 d 55 8

Physical Chemistry
Generali and i as shown in the diagram If ratio of their radil is concentric circular loops carrying current MEC 1 2 and ratio of the magnetic field due to A and B at the centre is 1 3 then the ratio of 4 1 1 2 C 13 will be 2 3 4 Loops 1 2 3 carry same current Torque on loon 1 is Class XIII Spartan batch Electronics Modern Physics Vector 1 0 Projectile fergen sefla en 1 12 fef 1 1 2 y 3 A 4452 6 F B A BI 1 21 A 1 3 11 12 11 12 2 12 XY1 1X2 B2

Physical Chemistry
General2 4 ms 3 15 ms 1 2 4 sinzo x8 xXx 2 4 ms gax 2 XXXsing 4 3 ms xxx 4 3 ms 3 15 ms ms 26 From the top of a tower of height 40 m a ball is projected 126 40 m HR20 m s 30 upwards with a speed of 20 m s at an angle of elevation of 30 Then the ratio of the total time taken by the ball to hit the ground to the time taken to ball come at same level as top of tower R M 844 3 20 24 sino uso rom 1 2 1 2x sino 2 3 1 FAR 1 2 1 y nmax 20m u w50 2h Raufan Uw 146 2 3 1 P Unus y R Z

Physical Chemistry
General9 Identify a N the following as atoms molecules or formula units b Co c CO d NaCl e H O 10 Find the molar mass of the following a H SO4 b Ca NO3 2